Vol 65, No 3 (2020)
- Year: 2020
- Published: 22.07.2020
- Articles: 7
- URL: https://virusjour.crie.ru/jour/issue/view/43
Full Issue
REVIEWS
RETRACTED: High-throughput sequencing in diagnostics and prevention of herpes simplex virus infection (Herpesviridae, Alphaherpesvirinae, Simplexvirus, Human alphaherpesvirus 1)
Abstract
Herpes simplex viruses types 1 (HSV-1) and 2 (HSV-2) are among the most common viruses in the human population. The clinical manifestations of HSV infection vary widely, which necessitates reliable molecular methods for the timely diagnosis of herpes virus infection, as well as for detection of mutations in the genes responsible for drug resistance. PCR is often unable to detect HSV isolates with nucleotide substitutions at the primer binding site. Sanger sequencing of the whole genome reveals mutations mainly at the consensus level, which accumulate at advanced stages of viral infection. High-throughput sequencing (HTS, next generation sequencing) offers an obvious advantage both in early diagnosis of herpes virus infection and identification of HSV variants.
 126-131
126-131


ORIGINAL RESEARCHES
Line immunoassay for detection of IgG antibodies to hepatitis E virus (Hepeviridae, Orthohepevirus, Orthohepevirus A)
Abstract
Introduction. The diagnostic efficacy of methods for hepatitis E serodiagnostic varies over a wide range; therefore, the combined use of tests of various formats is recommended. The aim of the research was to develop a test system for the detection of IgG antibodies to hepatitis E virus (HEV) in human serum by linear immunoassay (LIA).
Material and methods. Serum samples from patients with hepatitis and healthy individuals were tested using commercial enzyme-linked immunosorbent assay systems for the presence of IgG antibodies to viral agents causing hepatitis and other infections associated with liver pathology. Recombinant antigens ORF2 and ORF3 of HEV genotypes 1 and 3 were used. The “RecomLine HEV IgG/IgM” reagent kit (Mikrogen GmbH, Germany) was used as a comparison test system.
Results. The first Russian diagnostic kit “Blot-HEV”, designed to detect IgG antibodies to individual HEV proteins in human serum using LIA, was developed. The antigenic base is represented by strips of a nitrocellulose membrane with immobilized recombinant antigens ORF2 (aa 406–660) and ORF3 (aa 1–113) of HEV genotypes 1 and 3, and control antigens in the form of discrete lines. The conjugate was mouse monoclonal antibodies to human class G immunoglobulins labeled with horseradish peroxidase. The chromogen solution contained the 3,3’,5,5’-tetramethylbenzidine. A visual and digital recording of results was provided. The analytical sensitivity of the test kit was 0.625 IU/ml for ORF2 antigens and 2.5 IU/ml for ORF3 antigens. The absence of the influence of endogenous interfering substances on the results of the analysis and the absence of cross-reactions with antibodies to hepatitis pathogens of the other etiologies had been shown. The sensitivity of the test system compared to the “RecomLine HEV IgG/IgM” kit was 92%, specificity 97%. Shelf life in condition of storage was determined to be 12 months.
Conclusions. The developed test can be used to confirm the results of ELISA in laboratory diagnosis of hepatitis E.
 132-142
132-142


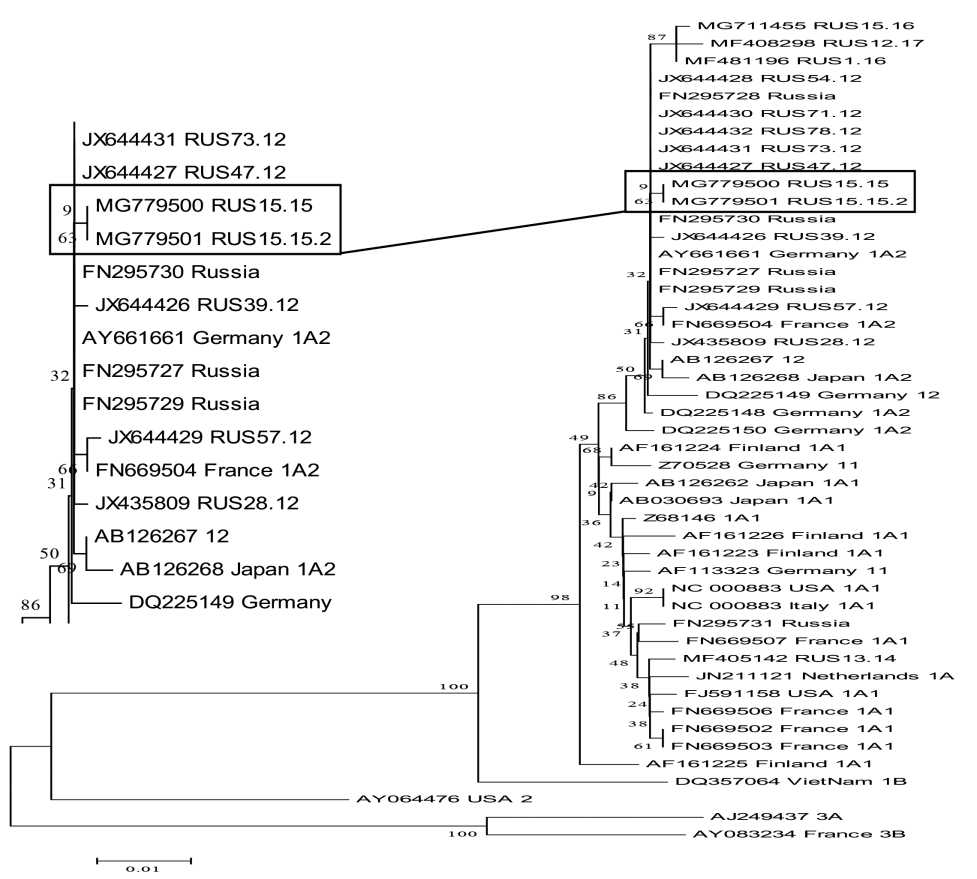
Results of a study of parvovirus B19 (Parvoviridae, Parvovirinae, Erythroparvovirus, Primate erythroparvovirus 1) prevalence and circulation activity in socially significant categories of the population
Abstract
Currently, along with the increasing need of medical organizations for blood preparations, algorithms for laboratory testing of blood donors are not available for all infections with hemo-contact mechanism of transmission. A representative example is infection caused by parvovirus В19.
Purpose of the study. The article presents the results of the original study, the purpose of which was to study the prevalence of antibodies to parvovirus B19 and the activity of the circulation of this virus in socially important categories of the population.
Material and methods. The materials of the study were blood samples from blood donors of Saint Petersburg, as well as parvovirus В19 sequences isolated from DNA-positive plasma samples.
Results and discussion. According to the results of the laboratory examination, a high proportion of carriers of virus-specific IgG antibodies was found in studied group of donors, which confirms the previous infection of parvovirus B19 in them and illustrates the high prevalence of infection in this socially significant group. Based on the results of the blood preparations testing, the presence of parvovirus DNA В19 in a significant number of samples was determined by polymerase chain reaction method. This indicates an current parvovirus infection in the examined donors and points to a high epidemiological risk of the blood products obtained from them. Sequencing and phylogenetic analysis of a fragment of the VP1 gene demonstrated that the studied isolates belonged to А1 genotype and its subtype 1А2, which correlates with the genotypes of parvovirus В19 circulating in the European Union and Asia. In addition, two previously unknown В19 parvovirus isolates were isolated, the nucleotide sequences of which were deposited into the international GenBank database.
Conclusion. Based on the results of the study, it is justified to include testing of blood samples for markers of В19 parvovirus infection in existing algorithms of laboratory examination of donors, which will ensure prevention of hemo-contact infection of blood recipients with parvovirus В19.
 143-149
143-149


Vaccination with virus-like particles containing hemagglutinin protects the lungs of mice with postifluenza bacterial pneumonia: virological, microbiological and clinical data
Abstract
Introduction. Influenza is a severe viral disease, a frequent complication of which is a secondary bacterial pneumonia. Influenza vaccines prevent secondary bacterial complications. Virus-like particles are one of the promising areas for the development of new vaccines.
The aim of this work is to study the correlation of the pathomorphological characteristics of the lungs with clinical, virological, and microbiological markers of the disease at vaccination with virus-like particles (VLPs), containing hemagglutinin (HA) of influenza virus (HA-Gag-VLPs) in a murine model of secondary bacterial pneumonia induced by S. pneumoniae after influenza infection.
Material and methods. BALB/c mice were vaccinated with VLPs containing influenza HA. After 21 days, mice were infected with two strains of influenza viruses, homologous and non-homologous, and 5 days after viral infection, were infected with S. pneumoniae. The vaccination effect was evaluated by morphological, virological (titer of the virus in the lungs) and microbiological (titer of bacteria in the lungs) data, and was confirmed by clinical data (survival, change in body weight).
Results. Immunization with HA-Gag-VLPs, followed by infection with a homologous influenza virus and S. pneumoniae, reduced the area of foci of inflammation, inhibited the replication of the virus and bacteria in the lungs, and also protected animals from death and reduced their weight loss. Immunization with HA-Gag-VLPs upon infection with a heterologous strain and S. pneumoniae did not affect these criteria.
Conclusion. The immunization with HA-Gag-VLPs prevented the viral replication, providing a reduction of S. pneumoniae titer and the degree of lung damage, protecting animals from the disease in a murine model of secondary bacterial pneumonia, induced by S. pneumoniae, after influenza infection with homologous strain of the virus.
 150-158
150-158


Modeling influenza virus infection in mature Wistar rats
Abstract
It has now been established that blood vessels are target for influenza, but the mechanism by which the influenza virus affects the cardiovascular system is unknown.
The aim – adaptation of influenza virus A/St. Petersburg/48/16 H1N1(pdm09) to mature Wistar rats, as these animals are the main experimental model for studying the pathology of the cardiovascular system.
Material and methods. Passage of influenza A virus (IAV) in embryonated chicken eggs, intranasal inoculation of rats with virus-containing material s, production of pulmonary homogenate, determination of IAV titer in embryonated chicken eggs, detection of histological changes in lung and pulmonary vessels.
Results. The article presents the results of the adaptation of influenza virus A/St. Petersburg/48/16 H1N1(pdm09) to mature Wistar rats. The infectious titer of the virus in the homogenates of infected rats lungs at the last stage of adaptation was 7.0 lg EID50/ml. Histological studies revealed pronounced changes in the respiratory tract (spasm of bronchioles, submucosal edema, desquamation of ciliated epithelium of bronchioles) and pulmonary vessels (spasm, desquamation and swelling of endotheliocytes, dissociation and swelling of the elastic membrane and media). In order to identify IAV in blood vessels and lung tissues, an immunohistochemical study was performed using monoclonal antibodies to NP antigen of IAV.
Conclusion. The data obtained allow us to conclude that the strain of influenza virus A/St. Petersburg/48/16 H1N1(pdm09) was adapted to mature Wistar rats maintaining virulent properties. The infectious titer of the virus at the last stage of adaptation was 7.0 lg EID50/ml. IAV identification is confirmed by immunohistochemical examination.
 159-166
159-166


DISCUSSION
The use of viral RNA polymerase inhibitors in combination with a fusion inhibitor in the treatment of patients with COVID-19: hypothesis
Abstract
This review article considers the possibilities of combined antiviral therapy in the treatment of patients with COVID-19, based on the analysis of the mechanism of action of known antiviral drugs in the framework of the medical hypothesis. The potential effectiveness of the joint use of viral RNA polymerase inhibitors and a fusion inhibitor in this pathology is discussed. The review discusses the main representatives of these groups of drugs – ribavirin, riamilovir, umifenovir, favipiravir. The efficacy and safety profile of these drugs was analyzed, including the experience of their use in clinical trials conducted during the COVID-19 pandemic, as well as earlier work performed during the SARS and MERS epidemics.
 167-175
167-175


OBITUARY
 176
176