In situ gels as a modern method of intranasal vaccine delivery
- Authors: Bakhrushina E.O.1, Mikhel J.B.1, Kondratieva V.M.2, Demina N.B.1, Grebennikova T.V.2
-
Affiliations:
- I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia
- National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya of the Ministry of Health of Russia
- Issue: Vol 67, No 5 (2022)
- Pages: 395-402
- Section: REVIEWS
- URL: https://virusjour.crie.ru/jour/article/view/645
- DOI: https://doi.org/10.36233/0507-4088-139
- ID: 645
Cite item
Abstract
The continuous emergence of new pathogens and the evolution of microbial drug resistance make it absolutely necessary to develop innovative, effective vaccination strategies. Use of nasal vaccination can increase convenience, safety, cause both local and systemic immune reactions. Intranasal administration nevertheless has a number of shortcomings that can be overcome by using the latest achievements of pharmaceutical science. One of the aspects of such solution may be the use of systems for the production of intranasal vaccines in situ – polymer compositions that provide a directed sol-gel transition controlled by the physiological conditions of the nasal cavity. At the same time, the gelation of the administered dose in contact with the nasal mucosa involves prolonged exposure of the drug at the injection site, greater mucoadhesion, counteraction to mucociliary clearance, modified and more complete release. A number of both foreign and domestic manufacturers produces polymers such as chitosan, gums, polyoxyethylene and polyoxypropylene block copolymers (poloxamers, proxanols), carbomers. For effective pharmaceutical development of new intranasal IBD delivery systems corresponding to the QbD concept, not only the knowledge of the range of excipients is necessary, but also simple, accessible, and reproducible methods for determining indicators that define the critical parameters of such delivery systems. In accordance with the conducted scientific search, the main indicators of standardization of in situ intranasal systems were identified: temperature and time of gel formation, gel strength, rheological characteristics, mucoadhesion, release, nasal mucociliary clearance time.
Full Text
Introduction
The most effective methods of prevention and treatment of infectious diseases are vaccination and immunobiological drugs (IBD). Notably, vaccines delivered intranasally have the capacity to induce immunity of the mucous membrane, tissues of which contain both antigen-presenting cells and antigen-processing cells capable of initiating cell-mediated immune responses, including immunological memory. Nasal vaccines can also induce systemic immunity through the mucosal immune system [1].
Intranasal vaccination was first mentioned in the article of the Soviet scientist Kh.M. Rosenberg in 1954 in the PubMed database of medical publications [2]. The first IBD used intranasally in living organisms is the BCG vaccine for tuberculosis [3].
The recently developed approach holding a lot of promise for development of vaccines employs VLPs (virus-like particles), which are made up of specific viral proteins and are spontaneously assembled into configurations imitating the conformational structure of viruses, but without viral genes. Intranasal vaccination with VLPs for prevention of influenza induces higher levels of cross-reactive IgA and IgG antibodies than parenteral vaccination. The preclinical studies have demonstrated that such vaccines tend to enhance both humoral and cell-mediated immune responses.
Currently, there are intranasal vaccines that have been approved or are being evaluated through clinical trials in Russia (Gam-COVID-Vac, VLP-Corona), the United States (AdCOVID, MV-014-212) and countries of the European Union (COVI-VAC, ChAdOx1-S). Vaccines injected intramuscularly are intended for inducing humoral and cell-mediated immune responses. However, they provide poor protection against replication of the virus in the upper respiratory tract due to the absence of the local IgA (sIgA) antibody immune response. At the moment, a number of new nasal vaccines against COVID-19 are being developed; the preclinical studies and clinical trials have demonstrated high-level production of neutralizing antibodies and IgA and T cell-mediated responses of mucous membranes.
Intranasal vaccine delivery offers multiple advantages compared to traditional injection routes: absence of invasion, high vascular density in the nasal cavity, capability of the therapeutic agent to reach cerebrospinal fluid, bypassing the blood-brain barrier. The downside of the intranasal route of delivery is mucociliary clearance playing a critical role in prompt removal of foreign substances from the nasal cavity [4]. Another physiological characteristic of the mucous membrane of the nasal cavity – active clearance driven by the coordinated beating of cilia that move foreign items and therapeutic agents trapped in the mucus out of the nose as well as multiple enzymes and specific interferon, which, in its turn, protects the body against pathogens, add up the complexity of intranasal vaccination [5]. However, these problems can be solved with the proper composition of additives to increase adhesion of the therapeutic agent on the mucous membrane and improve its full penetration into the systemic circulation [6]. Using of in situ delivery systems is one of the ways to solve the problems associated with intranasal IBD delivery.
In situ systems based on smart polymers are advanced systems for controlled delivery, which undergo phase changes in response to specific stimuli at the intended absorption site (the pH value, presence of specific ions, moisture, etc.).
Thus, the purpose of this study can be defined as a review of specific features and main aspects of pharmaceutical development of in situ systems for intranasal delivery of IMD.
In situ templates for intranasal vaccine delivery
A variety of polymers with different mechanisms of gelation and phase transition stimuli are used for development of in situ systems. These polymers can be natural (chitosan, pectin, gums) and synthetic (poloxamers, carbopols, polyvinyl alcohol, etc.).
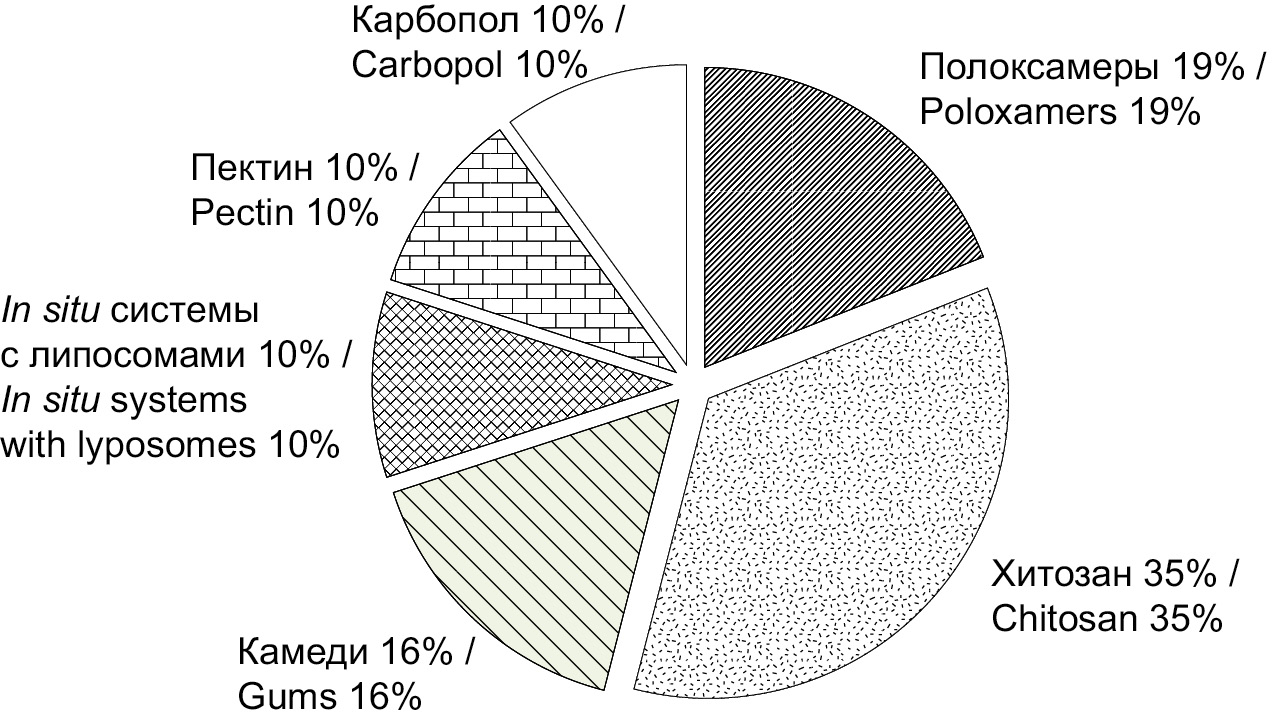
The analysis of the PubMed database of medical publications for the 2000–2022 timespan, using such keywords as intranasal in situ vaccine, intranasal in situ peptide, intranasal in situ protein, was used to estimate the usage frequency for different polymers in development of in situ IBD delivery systems (Figure). Natural polymers – chitosan (35%) and various gums (16%) as well as synthetic poloxamers (19%) were most popular. The main characteristics of polymers are discussed below.
Рисунок. Соотношение использования полимеров на основе анализа базы данных медицинских публикаций PubMed.
Chitosan is a naturally occurring bio-polysaccharide of cationic nature. Its mucoadhesion can be explained by the electrostatic interaction between its positively charged cationic molecule and negatively charged mucin [7–20].
Polyanions and polycations (such as chitosan) are characterized by the capacity for multipoint cooperative interaction with immunocompetent cells and can be seen as prospective immunomodulators [21].
Poloxamers are a class of nonionic triblock copolymers [9, 10, 15–17, 22, 23]. Poloxamers 188, 407, 124 are most frequently used in pharmaceutical IBD technology.
The studies of poloxamer 407 aqueous solutions demonstrated the occurrence of an in situ sol-gel transition at increased temperatures due to reduction in intermolecular interactions [24].
Poloxamer 188 is a safe biocompatible polymer, which can be used in the protein drug delivery system [25]. The pharmacological activity of poloxamer 188 has been extensively studied in the last ten years; it has been proved that it can be used in treatment of sickle cell disease [26]. Note that poloxamer 188 has hemorheological, antithrombic, anti-inflammatory properties, which are being extensively studied at present [27].
Among the above-mentioned triblock copolymers, poloxamer 124 can be used as a solubilizer, plasticizer and emulsifier; however, its in vivo pharmacokinetic properties are still unclear [28, 29].
As noted previously, poloxamer 407 is the main thermosensitive ingredient. Moreover, other excipients added to the complex that contains poloxamer 407 can be used to regulate the temperature of the phase transition and to increase the stability of the composition and to improve the biopharmaceutical properties [30].
Currently, a poloxamer 188 analog is manufactured in Russia, but no commercially manufactured alternative to the thermosensitive poloxamer 406 has been available so far [31, 32].
Three kinds of gums – gellan, xanthan and guar – have been studied as prospective components of in situ systems [33–37]. Gums used in such systems are responsible for ion-selective phase transition [38].
Gellan gum is an extracellular water-soluble anionic polysaccharide produced by bacteria Sphingomonas elodea [39].
Guar gum is a galactomannan polysaccharide extracted from guar beans.
Xanthan gum is a natural polysaccharide produced by fermentation of the gram-negative bacterium Xanthomonas campestris.
Pectin is an anionic biopolymer widely used in the food industry [40–42]. Natural pectin has anti-carcinogenic properties and can suppress colon cancer [43]. Largest manufacturers and markets for food and pharmaceutical pectin are concentrated in Europe, South America, China, and Iran. Currently, there is no commercial production of pectin in Russia [44].
Carbomers are high-molecular-weight crosslinked polyacrylic acid polymers [23, 45]. In healthcare practice, aqueous solutions of carbomers are used vaginally as a spermicide that protects against HIV infection and possibly other sexually transmitted diseases [46].
Development of intranasal in situ delivery systems for immunobiological drugs
The main disadvantage of regular nasal gels is the lack of dose precision; patients complain of the sensation of a foreign body in their nose. Addressing the problems, scientists from the Chinese University of Hong Kong have developed a thermoreversible in situ gel for intranasal delivery of the DB213 inhibitor of HIV-1 replication, using a combination of poloxamers 407, 188 and chitosan [16].
One of the first studies of intranasal in situ IBD delivery, including the in vivo testing of the formulation, was performed by Indian scientists Shailja et al. who developed a liposome in situ gelling system for delivery of hepatitis B vaccine [47]. The liposome medium was prepared from egg lecithin and cholesterol, while the polyacrylic acid served as an in situ polymer. The in vitro measurements showed that 54% of the drug was released, though the in vivo studies revealed excellent mucoadhesive properties of the formulation.
In 2020, Bedford et al. developed an in situ vaccine against influenza, using chitosan and poloxamers 188 and 407 as polymers responsible for its in situ properties [10]. The improved mucoadhesion achieved, in the authors’ opinion, by using chitosan was proved both in vitro and in in vivo tests.
U.S. researchers developed a GelVac® dry powder norovirus vaccine [40, 41]. The inactivated H5N1 influenza vaccine based on the GelVac® nasal powder formulation was approved by FDA (U.S. Food and Drug Administration) for clinical trials involving people. The tests using guinea pigs demonstrated enhanced and longer immunization compared to the non-in situ vaccines. The immune response resulted from the increased IgA levels in blood; the enzyme-linked immunosorbent assay (ELISA) was used for measurement of IgG1 and IgG2 subclass antibodies in pooled serum samples from each group.
At the Gamaleya National Research Center of Epidemiology and Microbiology of the Health Ministry of Russia, researchers tested the adjuvant for the VLP-based vaccine for intranasal administration to prevent COVID-19. The adjuvant consisted of the gel resulting from the mixture of gellan gum 0.5 and 2% poloxamer 124 diluted in distilled water with addition of 15% PBS (phosphate buffered saline). The trial tests showed that the gel demonstrated a high retention rate in the nasal cavity – up to 83%. The group of hACE2 AC70 mice intranasally immunized with the VLP-based vaccine containing 80 µg of the antigen per dose and the above adjuvant in the ratio 3 : 2 demonstrated the enhanced T cell-mediated immune response manifested in a statistically significant increase in the specific proliferation index (3.3 ± 0.28) in the blastogenic lymphocyte response, which correlated with production of interferon-γ secreting cells. After the animals had been infected with SARS-CoV-2, half of the animals who were vaccinated intranasally with three doses of the vaccine survived, while in the control group of animals who were not vaccinated all the animals died. The safety of gel was assessed and confirmed. The three-dose intranasal immunization with the VLP-based vaccine with gel adjuvant had no effect on the body mass index and body weight gain during 42 days of the experiment. The food and water consumption levels in the group of immunized animals were higher compared to the control group.
The autopsy examination of hACE2 AC70 mice on the 7th day after the second and third immunization, including visual examination of the exterior body condition, internal organs and tissues, cranial cavity, thoracic, abdominal and pelvic cavities, skeleton and musculoskeletal system did not reveal any gross changes associated with the effect of the vaccine containing an adjuvant.
Undoubtedly, the composition of gels used for intranasal vaccines needs to be further improved to optimize the formulations and enhance the protective effect of vaccines.
Design of pharmaceutical development of in situ intranasal immunobiological drugs
The most fundamental achievement of present-day research and development (R&D) in the pharmaceutical technology and biotechnology is optimization and standardization of the pharmaceutical development process [48, 49]. One of the most widely used methods is construction of a design space. Such methods are actively used in development of solid pharmaceutical forms (Harrington’s desirability function, SeDeM expert system) [50, 51] and are documented in ICH Q8.
Thus, with all present-day options that can be used to ramp up R&D processes, researchers have two main tasks: the well-grounded choice of a pool of auxiliary substances used in development and critical parameters for the specific system as well as reproducible available methods offering the reliability of the obtained results.
Selection of excipients for developing new in situ intranasal IBD delivery systems can be based on scientific and patent search (Figure). The evaluation of effectiveness and survivability of immunobiological substances is an essential part of IBD pharmaceutical development [52].
Selection of critical parameters for in situ intranasal delivery systems: temperature and gelation time, rheological parameters, in vitro release, in vitro / ex vivo mucoadhesion, in vivo control of mucociliary clearance [6].
Most of the researchers use the method offered by Gilbert et al. in 1987 to measure the gelation temperature [53].
Similar methods are used to measure gelation time and gel strength, which are critical for assessment of the mucociliary clearance and its reduction [10, 11, 16–18, 22, 23, 54].
Testers with vertical diffusion cells known as Franz cells are often used for evaluation of the IBD release profile in formulations [4, 8, 9, 24, 34].
To measure in vitro the mucoadhesive strength of polymeric compositions after the in situ gelation in the nasal cavity, Canadian scientists from McMaster University offered to calculate the mucoadhesive strength by constructing a calibration curve, the relationship of the tensile strength and the end position of plates with 2% mucin solution and the composition of the tested polymers [13].
To enhance the precision of screening, some researchers measure the nasal mucociliary clearance time following the method offered by Zaki et al. in 2007 [55].
Conclusion
In situ systems for intranasal vaccine delivery makes it possible to achieve both local and systemic effect of IBD without skin penetration. Vast experience has been gained in R&D processes, preclinical and clinical studies of such systems used for delivery of protein and other particles. The scientific literature search has shown that thermoreversible compositions of polymers are the most popular solution for intranasal in situ IBD delivery, and ion-selective polymers can be an excellent alternative for further research and development of new in situ systems of intranasal delivery.
Contribution: Bakhrushina E.O., Grebennikova T.V. – development of the review concept, article writing; Mikhel I.B., Kondratieva V.M. – selection of literature sources, data analysis; Demina N.B., Grebennikova T.V. – peer review, approval of the article for publication.
Funding. The research was funded by the state budget.
Conflict of interest. Authors declare no potential conflicts of interest.
About the authors
Elena O. Bakhrushina
I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia
Author for correspondence.
Email: bakhrushina_e_o@staff.sechenov.ru
ORCID iD: 0000-0001-8695-0346
PhD, Associate Professor, Associate Professor of the department of Pharmaceutical Technologies
Russian Federation, 119048, MoscowJoseph B. Mikhel
I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia
Email: mikheliosif@gmail.com
ORCID iD: 0000-0002-2866-0049
Student of the 5th year of the OD of the Institute of Pharmacy. A.P. Nelyubina
Russian Federation, 119048, MoscowValeria M. Kondratieva
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya of the Ministry of Health of Russia
Email: 1999valeriak@mail.ru
ORCID iD: 0000-0001-9163-4516
Graduate student
Russian Federation, 123098, MoscowNatalia B. Demina
I.M. Sechenov First Moscow State Medical University of the Ministry of Health of Russia
Email: demina_n_b@staff.sechenov.ru
ORCID iD: 0000-0003-4307-8791
Doctor of Pharmaceutical Sciences, Professor, Professor at the department of Pharmaceutical and Biomedical Technology
Russian Federation, 119048, MoscowTatyana V. Grebennikova
National Research Center of Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya of the Ministry of Health of Russia
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
Doctor of Biological Sciences, Professor, Corresponding Member RAS, Head Laboratory of Molecular Diagnostics, Head of department of Molecular Vaccinology and Immunodiagnostics
Russian Federation, 123098, MoscowReferences
- Xu H., Cai L., Hufnagel S., Cui Z. Intranasal vaccine: Factors to consider in research and development. Int. J. Pharm. 2021; 609: 121180. https://doi.org/10.1016/j.ijpharm.2021.121180
- Ivanov B.A. Russian literature on microbiology, immunology, infectious diseases and epidemiology published during the final months of 1954 and during the first quarter of 1955. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 1955; 32(12): 97–105. (in Russian)
- Rozenberg Kh.M. Experimental studies on intranasal BCG vaccination. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 1954; 31(7): 75–81. (in Russian)
- Porfir’eva N.N., Semina I.I., Mustafin R.I., Khutoryanskiy V.V. Intranasal administration as a route to deliver drugs to the brain (review). Razrabotka i registratsiya lekarstvennykh sredstv. 2021; 10(4): 117–27. https://doi.org/10.33380/2305-2066-2021-10-4-117-127
- Kunel’skaya N.L., Artem’eva-Karelova A.V. The main components of nasal secretions: mucoactive drugs. Lechebnoe delo. 2013; (3): 5–7. (in Russian)
- Bakhrushina E.O., Demina N.B., Shumkova M.M., Rodyuk P.S., Shulikina D.S., Krasnyuk I.I. In situ intranasal delivery systems: application prospects and main pharmaceutical aspects of development (review). Razrabotka i registratsiya lekarstvennykh sredstv. 2021; 10(4): 54–63. https://doi.org/10.33380/2305-2066-2021-10-4-54-63 (in Russian)
- Ivanushko L.A., Solov’eva T.F., Zaporozhets T.S., Somova L.M., Gorbach V.I. Antibacterial and antitoxic properties of chitosan and its derivatives. Tikhookeanskiy meditsinskiy zhurnal. 2009; (3): 82–5. (in Russian)
- Kempe S., Mäder K. In situ forming implants – an attractive formulation principle for parenteral depot formulations. J. Control. Release. 2012; 161(2): 668–79. https://doi.org/10.1016/j.jconrel.2012.04.016
- Vigani B., Rossi S., Sandri G., Bonferoni M.C., Caramella C.M., Ferrari F. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics. 2020; 12(9): 859. https://doi.org/10.3390/pharmaceutics12090859
- Bedford J.G., Caminschi I., Wakim L.M. Intranasal delivery of a chitosan-hydrogel vaccine generates nasal tissue resident memory CD8+ t cells that are protective against influenza virus infection. Vaccines (Basel). 2020; 8(4): 572. https://doi.org/10.3390/vaccines8040572
- Ozbılgın N.D., Saka O.M., Bozkır A. Preparation and in vitro/in vivo evaluation of mucosal adjuvant in situ forming gels with diphtheria toxoid. Drug Deliv. 2014; 21(2): 140–7. https://doi.org/10.3109/10717544.2013.834754
- Zhao K., Shi X., Zhao Y., Wei H., Sun Q., Huang T., et al. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine. 2011; 29(47): 8549–56. https://doi.org/10.1016/j.vaccine.2011.09.029
- Majcher M.J., Babar A., Lofts A., Leung A., Li X., Abu-Hijleh F., et al. In situ-gelling starch nanoparticle (SNP)/O-carboxymethyl chitosan (CMCh) nanoparticle network hydrogels for the intranasal delivery of an antipsychotic peptide. J. Control. Release. 2021; 330: 738–52. https://doi.org/10.1016/j.jconrel.2020.12.050
- Agrawal A.K., Gupta P.N., Khanna A., Sharma R.K., Chandrawanshi H.K., Gupta N., et al. Development and characterization of in situ gel system for nasal insulin delivery. Pharmazie. 2010; 65(3): 188–93.
- Luppi B., Bigucci F., Mercolini L., Musenga A., Sorrenti M., Catenacci L., et al. Novel mucoadhesive nasal inserts based on chitosan/hyaluronate polyelectrolyte complexes for peptide and protein delivery. J. Pharm. Pharmacol. 2009; 61(2): 151–7. https://doi.org/10.1211/jpp/61.02.0003
- Wang Q., Wong C.H., Chan H.Y.E., Lee W.Y., Zuo Z. Statistical Design of Experiment (DoE) based development and optimization of DB213 in situ thermosensitive gel for intranasal delivery. Int. J. Pharm. 2018; 539(1-2): 50–7. https://doi.org/10.1016/j.ijpharm.2018.01.032
- Ahmad N., Ahmad R., Ahmad F.J., Ahmad W., Alam M.A., Amir M., et al. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J. Biol. Sci. 2020; 27(1): 500–17. https://doi.org/10.1016/j.sjbs.2019.11.008
- Díaz A.G., Quinteros D.A., Gutiérrez S.E., Rivero M.A., Palma S.D., Allemandi D.A., et al. Immune response induced by conjunctival immunization with polymeric antigen BLSOmp31 using a thermoresponsive and mucoadhesive in situ gel as vaccine delivery system for prevention of ovine brucellosis. Vet. Immunol. Immunopathol. 2016; 178: 50–6. https://doi.org/10.1016/j.vetimm.2016.07.004
- Zadeh S.N., Rajabnezhad S., Zandkarimi M., Dahmardeh S., Mir L., Darbandi M.A., et al. Mucoadhesive microspheres of chitosan and polyvinyl alcohol as a carrier for intranasal delivery of insulin: in vitro and in vivo studies. MOJ Bioequiv. Availab. 2017; 3(2): 00030.
- Krauland A.H., Guggi D., Bernkop-Schnürch A. Thiolated chitosan microparticles: a vehicle for nasal peptide drug delivery. Int. J. Pharm. 2006; 307(2): 270–7. https://doi.org/10.1016/j.ijpharm.2005.10.016
- Das S.S., Kar S., Singh S.K., Hussain A., Verma P.R.P., Beg S. Chapter 13: Carboxymethyl chitosan in advanced drug-delivery applications. In: Hasnain M.S., Beg S., Nayak A.K., eds. Chitosan in Drug Delivery. Academic Press; 2022: 323–60. https://doi.org/10.1016/B978-0-12-819336-5.00006-6
- Park J.S., Oh Y.K., Yoon H., Kim J.M., Kim C.K. In situ gelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNA. J. Biomed. Mater. Res. 2002; 59(1): 144–51. https://doi.org/10.1002/jbm.1227
- Mura P., Mennini N., Nativi C., Richichi B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018; 122: 54–61. https://doi.org/10.1016/j.ejpb.2017.10.008
- Otero-Espinar F.J., Fernández-Ferreiro A., González-Barcia M., Blanco-Méndez J., Luzardo A. Chapter 6: Stimuli sensitive ocular drug delivery systems. In: Grumezescu A.M., ed. Drug Targeting and Stimuli Sensitive Drug Delivery Systems. William Andrew Publishing; 2018: 211–70. https://doi.org/10.1016/B978-0-12-813689-8.00006-9
- Tian J.L., Zhao Y.Z., Jin Z., Lu C.T., Tang Q.Q., Xiang Q., et al. Synthesis and characterization of Poloxamer 188-grafted heparin copolymer. Drug Dev. Ind. Pharm. 2010; 36(7): 832–8. https://doi.org/10.3109/03639040903520983
- Zylke J. Poloxamer 188 for Sickle Cell Disease. JAMA. 2021; 325(15): 1524. https://doi.org/10.1001/jama.2021.3399
- Emanuele M., Balasubramaniam B. Differential effects of commercial-grade and purified poloxamer 188 on renal function. Drugs R.D. 2014; 14(2): 73–83. https://doi.org/10.1007/s40268-014-0041-0
- Li Y., Cui Y., Li L., Lin X., Zhou X., Zhu H., et al. A UHPLC-Q-TOF/MS method for the determination of poloxamer 124 and its application in a tissue distribution study in rats. Anal. Methods. 2021; 13(45): 5516–22. https://doi.org/10.1039/d1ay01373d
- Li Y., Cui Y., Li L., Lin X., Zhou X., Zhu H., et al. Ultra-high-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry method for quantifying polymer poloxamer 124 and its application to pharmacokinetic study. J. Sep. Sci. 2021; 44(20): 3822–9. https://doi.org/10.1002/jssc.202100552
- Bakhrushina E.O., Novozhilova E.V., Kashperko A.S., Sokolova A.V., Demina N.B., Krasnyuk I.I. Biopharmaceutical study of binary poloxamer systems as in situ drug delivery systems poloxamer polycomplexes: The study. Int. J. Appl. Pharm. 2022; 14(3): 162–5. https://doi.org/10.22159/ijap.2022v14i3.43930
- Arshintseva E.V., Pushkin S.Yu. Comparative study of acute toxicity of poloxamers with intravenous administration in outbred rats. Internauka: nauchnyy zhurnal. 2022; 13(236). https://doi.org/10.32743/26870142.2022.13.236.336593 (in Russian)
- Vorob’ev S.I. New approach toward clinical trial design of targeted agents. Rossiyskiy bioterapevticheskiy zhurnal. 2009; 8(3): 3–8. (in Russian)
- Kola M., Puri G.K., Unnisa M.T., Swapna J., Phanivarma K. Formulation, optimization and evaluation of rasagiline mesylate in situ nasal gel. Indo Am. J. Pharm. Res. 2018; 8(09): 1645–54.
- Bertram U., Bernard M.C., Haensler J., Maincent P., Bodmeier R. In situ gelling nasal inserts for influenza vaccine delivery. Drug Dev. Ind. Pharm. 2010; 36(5): 581–93. https://doi.org/10.3109/03639040903382673
- Thakkar J.H., Prajapati S.T. Formulation development and characterization of in-situ gel of Rizatriptan Benzoate for intranasal delivery. J. Drug Deliv. Ther. 2021; 11(1-S): 1–6.
- Bertram U., Bodmeier R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 2006; 27(1): 62–71. https://doi.org/10.1016/j.ejps.2005.08.005
- Cao S.L., Ren X.W., Zhang Q.Z., Chen E., Xu F., Chen J., et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int. J. Pharm. 2009; 365(1-2): 109–15. https://doi.org/10.1016/j.ijpharm.2008.08.042
- Demina N.B., Bakhrushina E.O., Bardakov A.I., Krasnyuk I.I. Design of intranasal dosage forms: biopharmaceutical aspects. Farmatsiya. 2019; 68(3): 12–7. (in Russian)
- Maia F.R., Correlo V.M., Oliveira J.M., Reis R.L. Chapter 32: Natural origin materials for bone tissue engineering: properties, processing, and performance. In: Atala A., Lanza R., Mikos A.G., Nerem R., eds. Principles of Regenerative Medicine (Third Edition). Academic Press; 2019: 535–58. https://doi.org/10.1016/B978-0-12-809880-6.00032-1
- Ball J.P., Springer M.J., Ni Y., Finger-Baker I., Martinez J., Hahn J., et al. Intranasal delivery of a bivalent norovirus vaccine formulated in an in situ gelling dry powder. PLoS One. 2017; 12(5): e0177310. https://doi.org/10.1371/journal.pone.0177310
- Velasquez L.S., Shira S., Berta A.N., Kilbourne J., Medi B.M., Tizard I., et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011; 29(32): 5221–31. https://doi.org/10.1016/j.vaccine.2011.05.027
- Dukovski B.J., Plantić I., Čunčić I., Krtalić I., Juretić M., Pepić I., et al. Lipid/alginate nanoparticle-loaded in situ gelling system tailored for dexamethasone nasal delivery. Int. J. Pharm. 2017; 533(2): 480–7. https://doi.org/10.1016/j.ijpharm.2017.05.065
- Giri T.K. 5-nanoarchitectured polysaccharide-based drug carrier for ocular therapeutics. In: Holban A.M., Mihai G.A. Nanoarchitectonics for Smart Delivery and Drug Targeting. William Andrew Publishing; 2016: 119–41. https://doi.org/10.1016/B978-0-323-47347-7.00005-7
- Iklasova A.Sh., Sakipova Z.B., Bekbolatova E.N. Pectin: composition, technology of production, application in food and pharmaceutical industry. Vestnik Kazakhskogo natsional’nogo meditsinskogo universiteta. 2018; (3): 243–6. (in Russian)
- Patil P.R., Salve V.K., Thorat R.U., Sadhana S. Formulation and evaluation of ion-sensitive in-situ nasal gel of Zolmitriptan. Int. J. Pharm. Pharm. Sci. 2015; (7): 478–86.
- Gaganjot K., Grewal J., Jyoti K., Jain U.K., Chandra R., Madan J. Chapter 15: Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. In: Grumezescu A.M., ed. Drug Targeting and Stimuli Sensitive Drug Delivery Systems. William Andrew Publishing; 2018: 567–626. https://doi.org/10.1016/B978-0-12-813689-8.00015-X.
- Tiwari S., Goyal A.K., Mishra N., Vaidya B., Mehta A., Dube D., et al. Liposome in situ gelling system: Novel carrier based vaccine adjuvant for intranasal delivery of recombinant protein vaccine. Procedia Vaccinol. 2009; 1(1): 148–63. https://doi.org/10.1016/j.provac.2009.07.027
- Brkich G.E., Pyatigorskaya N.V., Kargin V.S., Zyryanov O.A. Development of the design of a study to determine the efficiency and safety of innovative drug. Mediko-farmatsevticheskiy zhurnal «Pul’s». 2022; 24(5): 19–23. https://doi.org/10.26787/nydha-2686-6838-2022-24-5-19-23 (in Russian)
- Zyryanov O.A. Development of the composition and technology for obtaining a dosage form based on triazatricyclotetradecane potential modulator of the AMPA receptor: Diss. Moscow; 2021. (in Russian)
- Flórez Borges P., García-Montoya E., Pérez-Lozano P., Jo E., Miñarro M., Manich A., et al. The role of SeDeM for characterizing the active substance and polyvinyilpyrrolidone eliminating metastable forms in an oral lyophilizate-A preformulation study. PLoS One. 2018; 13(4): e0196049. https://doi.org/10.1371/journal.pone.0196049
- Gulenkov A.S., Mizina P.G., Bakhrushina E.O., Bardakov A.I., Nyudochkin A.V. Рharmaceutical-technological study of adsorbed liquid plant extract of antimicrobial activity. Razrabotka i registratsiya lekarstvennykh sredstv. 2022; 11(2): 94–101. https://doi.org/10.33380/2305-2066-2022-11-2-94-101 (in Russian)
- Bakhrushina E.O., Anurova M.N., Aleshkin A.V., Demina N.B., Krasnyuk I.I., Pyatigorskaya N.V., et al. Modern tendencies of the use and development of drugs of bacteriophages. Vestnik Rossiyskoy akademii meditsinskikh nauk. 2021; 76(4): 351–60. https://doi.org/10.15690/vramn1380 (in Russian)
- Gilbert J.C., Richardson J.L., Davies M.C., Palin K.J., Hadgraft J. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. J. Control. Release. 1987; 5(2): 113–8. https://doi.org/10.1016/0168-3659(87)90002-2
- Nižić L., Ugrina I., Špoljarić D., Saršon V., Kučuk M.S., Pepić I., et al. Innovative sprayable in situ gelling fluticasone suspension: Development and optimization of nasal deposition. Int. J. Pharm. 2019; 563: 445–56. https://doi.org/10.1016/j.ijpharm.2019.04.015
- Zaki N.M., Awad G.A., Mortada N.D., Elhady S.S.A. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with 28 modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007; 32(4-5): 296–307. https://doi.org/10.1016/j.ejps.2007.08.006