Modeling of mixed infection with Zika and West Nile viruses (Flaviviridae: Orthoflavivirus: Orthoflavivirus zikaense, Orthoflavivirus nilense) in vitro and in vivo
- Authors: Svyatchenko V.A.1, Protopopova E.V.1, Legostaev S.S.1, Mikryukova T.P.1, Agafonov A.P.1, Loktev V.B.1
-
Affiliations:
- State Research Center of Virology and Biotechnology «Vector»
- Issue: Vol 70, No 4 (2025)
- Pages: 340-348
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16766
- DOI: https://doi.org/10.36233/0507-4088-324
- EDN: https://elibrary.ru/gkebwv
- ID: 16766
Cite item
Abstract
Introduction. Mosquito-borne human diseases caused by Zika virus and West Nile virus (WNV) are widespread across multiple continents and cause major outbreaks. Their ranges overlap and the possibility of mixed infections is obvious. The information of such mixed infections is limited.
The aim of the study is to investigate the features of mixed infection of WNV and Zika virus in vitro and in vivo in order to assess their possible interference and/or enhancement of viral infection.
Materials and methods. The study used West Nile virus and Zika virus strains Vlg27924 and MR766, respectively. The infectious activity of viruses during mono- and co-infection was determined on Vero E6 cell culture using RT-PCR, as well as on BALB/c mice using various administration schemes.
Results. In vitro studies of co-infection with WNV and Zika virus showed that co-infection leads to interference, with the degree of competitive inhibition of replication being more pronounced for Zika virus, reaching 1000 times or more when compared to mono-infection. During simultaneous infection in mice, Zika virus does not affect the development of lethal infection caused by WNV. However, preliminary (4 and 20 days) infection with a sublethal dose of Zika virus reliably protects animals from subsequent administration of 10 and 100 LD50 WNV, respectively. In pre-infected and co-infected animals with Zika virus, the development of WNV-specific viral neutralizing antibodies was recorded in higher titers than in WNV monoinfected animals.
Conclusion. The presence of in vitro interference between the studied orthoflaviviruses was shown, most pronounced in relation to the Zika virus. No significant effect was observed with simultaneous co-infection in vivo. However, pre-infection of mice with Zika virus provides protection to animals from lethal WNV infection due to the induction of high levels of antibodies that specifically neutralize its infectious activity.
Keywords
Full Text
Introduction
Many RNA-containing orthoflaviviruses belonging to the Flaviviridae family (Riboviria; Orthornavirae; Kitrinoviricota; Flasuviricetes; Amarillovirale; Flaviviridae; Orthoflavivirus) cause severe human diseases [1]. Currently, orthoflaviviruses are widespread almost everywhere, forming natural infection foci that sometimes encompass entire regions and continents, as well as causing global outbreaks of human diseases [2, 3]. It is customary to distinguish the so-called major flavivirus infections, which are caused by dengue, Japanese encephalitis, West Nile (WNV), yellow fever, and Zika viruses, and with which tens of millions of human infections are associated [4]. The onset of the global outbreak of West Nile fever associated with WNV genotype 1a is related with outbreaks of the disease in 1999 in the USA and Russia [5]. Over the next few years, this infection spread to almost every continent [6–8]. The rapid spread of the infection has been linked to various species of wild birds and mosquitoes of the genus Culex. In the Russian Federation, WNV genotypes 1 and 2 constantly circulate in the central and southern European parts of Russia, in the south of Western Siberia and in the Far Eastern region [6, 7]. Culex pipiens mosquitoes, as well as C. tarsalis and C. quinquefasciatus, are considered the main vectors of WNV in various regions of the world.
Zika virus was originally isolated in Uganda in 1947 and was associated with Aedes mosquitoes [9]. Later, its circulation was described in countries of the Indian Ocean basin and in 1966 the Asian genotype of the Zika virus was isolated [10]. In fact, the widespread spread of Zika fever began in 2007, and in 2015–2016 the disease began to be registered in the countries of Central and South America. By the end of 2023, the infection had been reported in more than 92 countries, not counting countries, including Russia, where imported cases of the disease had been reported. The main vector for the spread of Zika fever is considered to be Aedes aegypti and Ae. albopictus mosquitoes, however, other mosquitoes of the genus Aedes may also be involved in the spread of the Zika virus [10].
Orthoflaviviruses, like other arboviral infection agents, can be transmitted through mosquito bites, which can be infected with several types of pathogens simultaneously [11]. Thus, Ae. aegypti and Ae. albopictus mosquitoes have been simultaneously infected with Zika, dengue and chikungunya viruses [12]. There are known cases of mixed infections with Zika/chikungunya, Zika/dengue, dengue/chikungunya and Zika/dengue/chikungunya in humans associated with mosquitoes [13–17]. The clinical picture of mixed infections caused by orthoflaviviruses may differ from mono-infections, making their diagnosis and treatment more difficult. It is important to note that for a number of mixed infections, modulation of virulence is unique to them, which can lead to an increase (or decrease) in the severity of the disease [18, 19]. Thus, sequential infection with different subtypes of the dengue virus can cause a sharp increase in the severity of the disease in humans, up to and including fatal cases [20]. This phenomenon is usually associated with the action of pre-existing antibodies to another subtype of the dengue virus, which causes an increase in the severity of recurrent dengue fever in humans.
The importance of the issue of mixed infections is due to the complexity of prevention, diagnosis, and treatment of such cases. At the same time, there is insufficient information about the occurrence, characteristics and consequences of mixed infections in different regions of the world [21].
The aim of this study is the experimental investigation of the characteristics of mixed infection with WNV and Zika virus in vitro and in vivo.
Materials and methods
The study experiments used WNV (strain Vlg27924) and Zika virus (strain MR766) obtained from the State Collection of Pathogenic Microorganisms and Rickettsiae of the State Research Center of Virology and Biotechnology «Vector». The viruses were cultured on monolayer cell cultures of Vero E6 and SPEV (swine embryonic kidney cell culture), grown to 80–90% confluency in DMEM F12 medium (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA), penicillin 100 IU/mL, and streptomycin 100 µg/mL (Gibco, USA), in an atmosphere with 5% CO2 at 37 °C. The infectious activity of the viral substances was determined by microtitration on 96-well culture plates (Greiner, Austria) with a subconfluent monolayer of cells, as previously described [22, 23]. The results were recorded with microscopy after 6 days based on the development of CPE (cytopathogenic effect) and/or using the MTT assay. The calculation of viral infectious titers was performed using the Spearman-Karber method and expressed as log10 TCID50 (50% tissue culture infectious dose). The viral substances were stored at a temperature of −80 °C.
The study of the effects of in vitro co-infection was conducted using Vero E6 cells with simultaneous and sequential (in different order) infection of cell cultures with WNV and Zika virus. Infected cell cultures (three repetitions per experimental point) in 24-well culture plates (Greiner, Austria) were incubated at 37 °C in a CO2 incubator, and samples were taken at different time intervals to determine viral load using reverse transcription polymerase chain reaction (RT-PCR) as we described earlier [24]. Mono-infected cell cultures were used as controls. RNA was extracted from samples using the Ribo-Prep RNA kit (Central Research Institute of Epidemiology of Rospotrebnadzor, Moscow). To detect viral RNA, reagent kits for identifying WNV and Zika virus RNAs (State Research Center of Virology and Biotechnology «Vector», Koltsovo, Novosibirsk region) were used by the RT-PCR method with hybridization-fluorescent detection in real-time mode, using primers specific to the 3’-UTR and NS5 gene, respectively. Specificity control was performed using viral suspensions containing 106 TCID50/mL of WNV or Zika virus. The absence of a significant effect of one virus on the quantitative detection of another in the mixture was confirmed by a model experiment (Fig. S1, Supplementary Materials). The registration of results was carried out using the CFX 96 device (Bio-Rad, USA). Standard curves were generated by 10-fold serial dilution of the internal positive control samples (IPCS) supplied with the respective PCR kit (from 106 to 0.1 copies/reaction). Ct values of samples were obtained from two fluorescence channels, for viral cDNA and for IPCS. Viral cDNA Ct values were scaled relative to IPCS Ct values [25].
Infection of BALB/c mice weighing 10–12 g was carried out by intraperitoneal administration of 200 µL of a virus-containing suspension, while control animals were administered an equivalent volume of saline solution. In the study of mixed infections, the following experimental schemes were used: mono-infection of animals with Zika virus and WNV; simultaneous infection with Zika virus and WNV; sequential infection (Zika virus and subsequently WNV after 4 days; Zika virus and subsequently WNV after 20 days). Mono-infected animals with either WNV or Zika virus were used as comparison. During the experiment, the condition of the animals was assessed daily. The animals were housed in same-sex groups in individually ventilated cages (Animal Care Systems) under controlled conditions, at a temperature of 22–26 °C and a relative humidity of 30–60%. Granulated feed and water were provided ad libitum to the animals.
The neutralization reaction (NR) of WNV or Zika virus was conducted using the micro-method on Vero E6 cells with double serial dilutions (starting from 1:10) of individual mouse sera, heated at 56 °C for 30 minutes [26]. The titers of virus-neutralizing antibodies were taken as the inverse values of the serum dilutions that completely neutralized the infectious activity of WNV or Zika virus at a dose of 50 TCID50.
Statistical analysis, including calculation of mean, standard deviation, and coefficient of variation of the mean Ct value, was performed using Excel (Microsoft Corp., USA). Statistical processing of data was also carried out using the statistical program STATISTICA 12 (StatSoft Inc., USA). Statistical assessment of differences between groups was performed using Student’s t-test; a p value < 0.05 was considered significant.
Experiments with infectious material were conducted in accordance with the requirements of biosafety regulations as stipulated in SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases” dated January 1st, 2021. Infection of mice with WNV and Zika virus along with the rest of the manipulations with them were carried out in the infectious vivarium of the State Research Center of Virology and Biotechnology «Vector» of Rospotrebnadzor under BSL-3 conditions.
Authors confirm compliance with institutional and national standards for the use of laboratory animals in accordance with «Consensus Author Guidelines for Animal Use» (IAVES, 23 July 2010). The study protocol was approved by the Bioethics Committee of the State Scientific Center of Virology and Biotechnology «Vector» of Rospotrebnadzor (Protocol No. 02 of April 03, 2023).
Results
Mixed infection in vitro. The replicative activity of viruses in vitro during mixed infection compared to mono-infection was assessed by determining viral load using quantitative PCR analysis (Table 1). Simultaneous infection led to pronounced inhibition of Zika virus replication, with no significant effect of coinfection on the accumulation of infectious viral particles in infected cells (Table 1 a). Sequential infection led to competitive inhibition of the viral infectivity, with the degree of competitive inhibition being significantly more pronounced for the Zika virus (Table 1 b). Thus, the viral load of the Zika virus in cells pre-infected with WNV and then infected with the Zika virus after 24 hours was more than 3 orders of magnitude lower than in the corresponding Zika virus mono-infected cells. In reverse experiments, the differences in viral load of WNV in cells with mixed-infection and mono-infection did not exceed 1.5 log10.
Table 1. Simultaneous and sequential infection of Vero E6 cell culture with West Nile virus (WNV) and Zika virus (MOI 0.5 TCID50)
Таблица 1. Одновременное и последовательное инфицирование культуры клеток Vero E6 вирусом Западного Нила (ВЗН) и вирусом Зика (МОИ 0,5 ТЦД50)
Simultaneous infection (а) Одновременное инфицирование (а) | ||||
Time post-infection Время после инфицирования | WNV (log10 RNA copies/mL) ВЗН (lg копий РНК/мл) | Zika virus (log10 RNA copies/mL) Вирус Зика (lg копий РНК/мл) | WNV/Zika virus (log10 RNA copies/mL) ВЗН/вирус Зика (lg копий РНК/мл) | |
24 h / ч | < 3.0 | < 3.0 | < 3.0/< 3.0 | |
48 h / ч | 4.3 ± 0.3 | 3.8 ± 0.2 | 4.0 ± 0.3/3.2 ± 0.2 | |
72 h / ч | 6.5 ± 0.4 | 5.7 ± 0.3 | 6.3 ± 0.4/3.5 ± 0.2 | |
96 h / ч | 8.1 ± 0.4 | 7.8 ± 0.3 | 7.7 ± 0.3/3.9 ± 0.3 | |
Sequential infection (b) Последовательное инфицирование (б) | ||||
WNV‒24 hours‒Zika virus ВЗН‒24 ч‒вирус Зика | PBS‒24 hours‒Zika virus PBS‒24 ч ‒вирус Зика | |||
Time post-infection with Zika virus Время после инфицирования вирусом Зика | WNV/ Zika virus (log10 RNA copies/mL) ВЗН/вирус Зика (lg копий РНК/мл) | Zika virus (log10 RNA copies/mL) Вирус Зика (lg копий РНК/мл) | ||
24 h / ч | 4.5 ± 0.3/< 3.0 | < 3.0 | ||
48 h / ч | 6.2 ± 0.4/3.2 ± 0.3 | 4.2 ± 0.2 | ||
72 h / ч | 7.4 ± 0.3/3.4 ± 0.3 | 6.9 ± 0.3 | ||
Zika virus‒24 hours‒WNV Вирус Зика‒24 ч‒ВЗН | PBS‒24 hours‒WNV PBS‒24 ч‒ВЗН | |||
Time post-infection with WNV Время после инфицирования ВЗН | Zika virus/WNV (log10 RNA copies/mL) Вирус Зика/ВЗН (lg копий/мл) | WNV (log10 RNA copies/mL) ВЗН (lg копий РНК/мл) | ||
24 h / ч | 3.5 ± 0.3/< 3.0 | < 3.0 | ||
48 h / ч | 5.1 ± 0.4/3.3 ± 0.3 | 4.2 ± 0.2 | ||
72 h / ч | 6.5 ± 0.4/5.0 ± 0.3 | 6.4 ± 0.2 | ||
Note. Values represent M ± SD of three independent experiments. Student's t-test was used to compare two groups.
Примечание. Значения представляют собой M ± SD трех независимых экспериментов. Для сравнения двух групп использовался t-критерий Стьюдента.
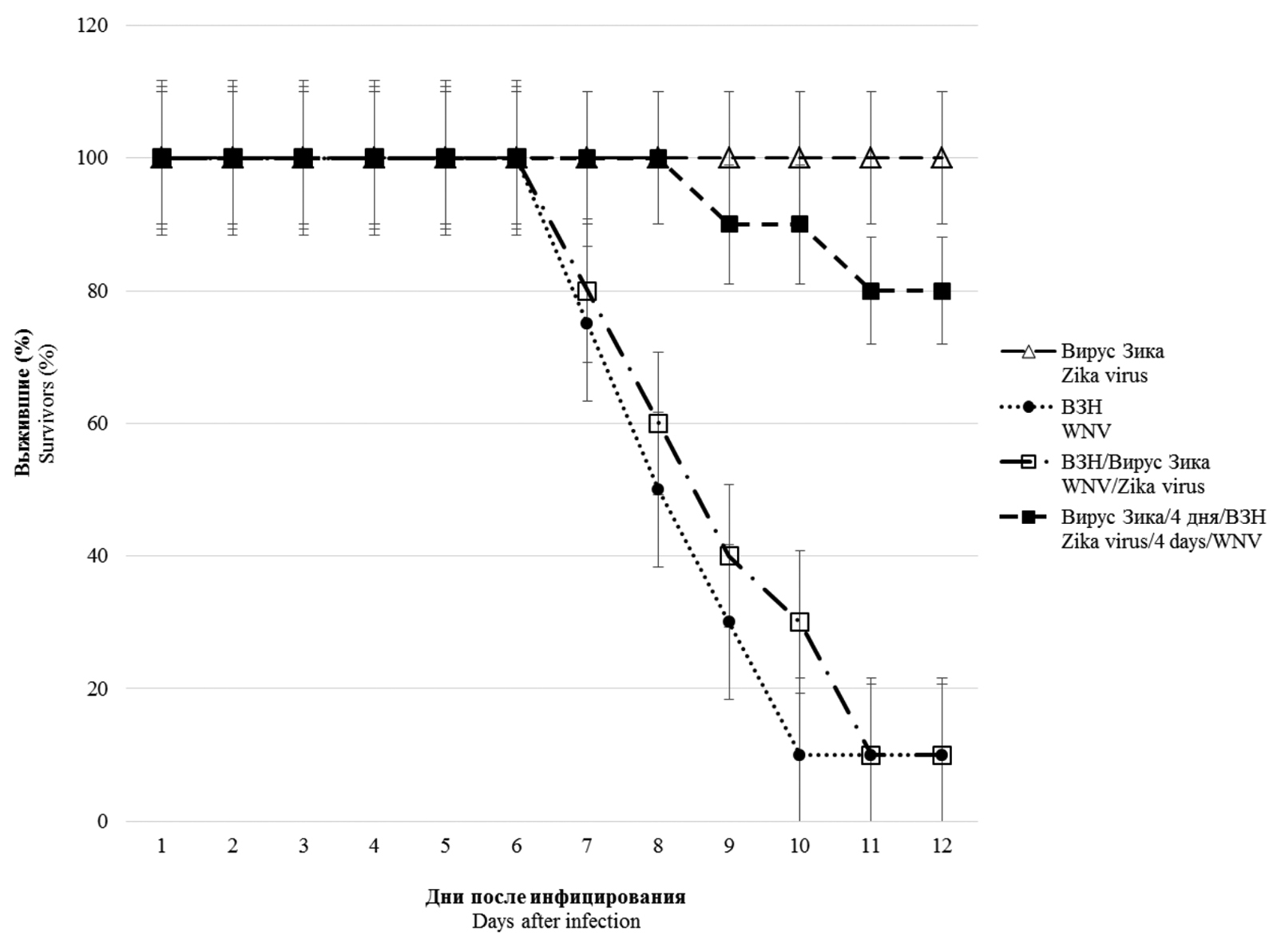
Mixed infection in vivo. Co-infection with WNV and Zika virus was modeled through simultaneous and sequential infection of mice with different doses of the viruses (Zika virus and WNV after 4 days; Zika virus and WNV after 20 days). The results presented in Fig. 1 show that infection with the Zika virus (104 TCID50/mouse) did not cause the development of a lethal infection, and no visible clinical manifestations of viral infection were observed in the animals. On the contrary, infection of mice with WNV at a dose of 103 TCID50 led to the death of 90% of the mice with the development of clinical symptoms of neuroinfection. In the case of mixed simultaneous infection with two viruses, the Zika virus did not significantly affect the course and outcome of the infectious process caused by WNV. At the same time, when animals were pre-infected with the Zika virus (4 days before being infected with WNV), 80% of the mice infected with 10 LD50 of WNV survived.
Fig. 1. Survival of BALB/c mice infected with West Nile virus (WNV) and Zika virus under different schemes of virus administration.
△ – experimental results obtained by intraperitoneal Zika virus infection with a dose of 104 TCID50; ● – experimental results obtained by intraperitoneal infection of WNV with a dose of 103 TCID50 (10 LD50); □ – experimental results obtained by simultaneous intraperitoneal infection with Zika virus (104 TCID50) and WNV (10 LD50); ■ – experimental results obtained by intraperitoneal infection with Zika virus (104 TCID50) and after 4 days intraperitoneal infection with 10 LD50 of WNV (n = 10; p < 0.05).
Рис. 1. Выживаемость мышей BALB/c, инфицированных вирусами Западного Нила (ВЗН) и Зика, при разных схемах введения вирусных препаратов.
△ – результаты, полученные при внутрибрюшинном инфицировании вирусом Зика дозой 104 ТЦД50; ● – при внутрибрюшинном инфицировании ВЗН дозой 103 ТЦД50 (10 ЛД50); □ – при одновременном внутрибрюшинном инфицировании вирусом Зика (104 ТЦД50) и ВЗН (10 ЛД50); ■ – при внутрибрюшинном инфицировании вирусом Зика (104 ТЦД50) и через 4 сут внутрибрюшинном инфицировании ВЗН (10 ЛД50) (n = 10; p < 0,05).
Figure 2 shows the results of infecting BALB/c mice 20 days after prior infection with the Zika virus at a dose of 104 TCID50/mouse. A 100% and 90% protection of Zika virus pre-infected animals from sequential infection with doses of 10 and 100 LD50 of WNV, respectively, was observed. These data indicate that prior infection with the Zika virus provides significant protection to animals against sequential infection with lethal doses of the Zika virus.
Fig. 2. Survival of Zika virus pre-infected BALB/c mice upon subsequent infection with lethal doses of West Nile virus (WNV).
△ – experimental results obtained by intraperitoneal infection with Zika virus (104 TCID50) and after 20 days intraperitoneal infection with WNV (10 LD50); ● – experimental results obtained by intraperitoneal infection with WNV (100 LD50); □ – experimental results obtained with intraperitoneal infection of WNV (10 LD50); ■ – experimental results obtained by intraperitoneal infection with Zika virus (104 TCID50) and after 20 days intraperitoneal infection with WNV (100 LD50) (n = 10; p < 0.05).
Рис. 2. Выживаемость предварительно инфицированных вирусом Зика мышей BALB/c при последующем инфицировании летальными дозами вируса Западного Нила (ВЗН).
△ – результаты, полученные при внутрибрюшинном инфицировании вирусом Зика (104 ТЦД50) и через 20 сут при внутрибрюшинном инфицировании ВЗН (10 ЛД50); ● – при внутрибрюшинном инфицировании ВЗН (100 ЛД50); □ – при внутрибрюшинном инфицировании ВЗН (10 ЛД50); ■ – при внутрибрюшинном инфицировании вирусом Зика (104 ТЦД50) и через 20 сут при внутрибрюшинном инфицировании ВЗН (100 ЛД50) (n = 10; p < 0,05).
Induction of virus-neutralizing antibodies in BALB/c mice. Individual sera from experimental animals were tested for the presence of virus-neutralizing antibodies (Table 2). Serum from mice infected with either Zika virus or WNV neutralized only homologous viruses. However, after being infected with WNV, the sera of animals previously infected with the Zika virus were able to neutralize both the Zika virus and WNV. At the same time, the titers of virus-neutralizing antibodies in sera that were pre-infected with the Zika virus and then infected with 100 LD50 of WNV were significantly higher than in animals infected only with WNV. To confirm the specificity of the neutralizing antibodies and the absence of their cross-reactivity in the serum, an assessment of their neutralizing activity against a heterologous virus was conducted. Thus, the sera of surviving mice after their infection with WNV at a dose of 10 LD50, 21 days post-infection, had a high titer of neutralizing antibodies against WNV (750 ± 107), but they were unable to neutralize the Zika virus in the cell culture.
Table 2. Induction of virus-neutralizing antibodies in BALB/c mice pre-infected with Zika virus and after 20 days infected with West Nile virus (WNV) (8 days after WNV infection)
Таблица 2. Индукция вируснейтрализующих антител у мышей BALB/c, инфицированных вирусом Зика и через 20 сут зараженных вирусом Западного Нила (ВЗН) (8 сут после заражения ВЗН)
Mouse serum Сыворотки мышей | Virus neutralizing antibody titers (M ± SEM) Титры вируснейтрализующих антител (M ± SEM) | |
Zika virus Вирус Зика | WNV ВЗН | |
Mono infection Моноинфекция | ||
Zika virus (20 days) Вирус Зика (20 сут) | 82.3 ± 15.4 | < 10 |
WNV (8 days)* ВЗН (8 сут)* | < 10 | 112.6 ± 27.2 |
Co-infection Коинфекция | ||
Zika virus/20 days/WNV (8 days) Вирус Зика/20 сут/ВЗН (8 сут) | 74.5 ± 17.7 | 525.7 ± 73.5 |
Control Контроль | < 10 | < 10 |
Note. * ‒ death of 100% of animals to tenth day after infection with 100 LD50 of WNV. The corresponding dilution of an individual animal serum neutralizing 50 TCID50 of Zika virus or WNV in the neutralization test was taken as a titer. Geometric mean titers of virus neutralizing antibodies are presented (n = 5; p < 0.05).
Примечание. * ‒ гибель 100% животных к 10-м суткам после инфицирования 100 ЛД50 ВЗН. За титр принимали соответствующее разведение
Discussion
The phenomenon of interference between different viruses in a susceptible cell has been known for quite some time and, in its classical form, suggests the suppression of one virus’s replication by another [27]. Possible mechanisms of this phenomenon are associated with various key points of viral replication in the cell, induction of antiviral defense systems in the cell, including interferon systems, competition for cellular resources for the synthesis of viral molecules and viral particles, different rates of viral replication, and a number of other factors. The entry of a viral particle into a cell can dramatically alter the functioning of the cellular genome, including or inhibiting various genes [28]. These changes can involve dozens or even hundreds of cellular genes. The complexity of conducting these studies is predetermined by our insufficient knowledge of the phenomenon of interference between different viruses, and the development of research on the genetic diversity of viromes has highlighted the urgent necessity to facilitate research development in this area [29]. The peculiarity of orthoflaviviruses is that in natural foci, they are usually spread by vectors [11]. Ixodid ticks and various species of mosquitoes are the most common vectors of these viruses. In the case of overlapping natural foci, mixed infections are possible in both invertebrates and their warm-blooded hosts. The Zika virus and WNV belong to the so-called mosquito-borne flaviviruses and often circulate in the same regions, so cases of co-infection are quite likely among susceptible host species, including humans.
In the experiments of the current study, Zika virus and WNV, which are widely distributed in the modern world and have recently caused nearly global outbreaks of diseases in countries with warm climates, were used. The results obtained using these viruses demonstrate the presence of in vitro interference between these two orthoflaviviruses. Simultaneous infection leads to pronounced inhibition of Zika virus replication, with no similarly pronounced effect on WNV replication in mammalian cells. Sequential (in different order) infection also leads to competitive mutual inhibition of the replication of these viruses, with the degree of competitive inhibition remaining more pronounced for the Zika virus. Recently, it was shown that coinfection of Vero cells with WNV and St. Louis encephalitis virus (SLEV) is accompanied by inhibition of SLEV replication with minimal effect on WNV replication activity. At the same time, coinfection of mosquito cells CT (Cx. tarsalis) did not affect the replication of any of the viruses [30].
Mixed infections in laboratory animal models (in vivo) partially confirmed the results obtained in the cell culture. Thus, simultaneous infection of BALB/c mice with the Zika virus did not significantly affect the course of the disease and the fatal outcome of the infectious process caused by WNV. However, when the animals were pre-infected with the Zika virus (4 days before being infected with WNV), a significant decrease in WNV infectious activity was observed in the laboratory animals. These results indicate the possibility of in vivo interference between these two flaviviruses. We were unable to assess the reverse effect due to the fact that the chosen animal infection model did not ensure the development of clinically apparent and/or lethal infection after the administration of the Zika virus. When modeling co-infection by infecting animals with WNV 20 days after Zika virus infection, it was found that prior infection with Zika virus almost completely protects the animals from subsequent infection with 10 and 100 LD50 of WNV.
In the current study, it was demonstrated that, unlike previously published data on mixed infections with Zika and dengue viruses [30, 31], WNV infection in mice pre-infected with Zika virus was not accompanied by an exacerbation of infection symptoms. Moreover, upon prior infection with the Zika virus, the development of significant protection in animals against subsequent infection with WNV was observed. The key role of neutralizing antibodies in protection against flavivirus infections has been previously demonstrated [32–34]. The assessment of the ability of serum samples to neutralize Zika virus and WNV was tested in a viral neutralization assay on Vero E6 cell culture. The induction of neutralizing antibodies was observed only against the homologous virus (Table 2), and cross-reactivity of neutralizing antibodies was absent. It is important to emphasize that prior infection with the Zika virus led to a more rapid response to WNV infection, enhancing the subsequent production of neutralizing antibodies against WNV. Moreover, the level of antibodies was sufficient to protect the animals from lethal infection. The ambiguity of potential outcomes of mixed infection with WNV and Zika virus is illustrated in [35], which showed that serum samples from WNV-seropositive individuals effectively neutralize WNV but enhance the infectious activity of Zika virus.
The biological significance of a prior non-lethal Zika virus infection, which provides protection against WNV, may limit the circulation of these orthoflaviviruses in the natural foci of these infections. These results may be useful for further studying mixed infections caused by Zika and WNV, as well as for the future development of broad-spectrum immunoprophylactic agents against flavivirus infections.
Conclusion
Thus, the study of mixed infection of two orthoflaviviruses in an in vitro model showed the presence of pronounced viral interference in the Vero E6 cell culture. Thus, WNV, when simultaneously and sequentially infected, inhibited Zika virus replication in cell culture by more than 1000 times, while the reverse effect did not exceed 30 (1.5 log10). Modeling mixed infection in vivo showed a more complex picture of the infectious process development in BALB/c mice. In simultaneous mixed infection of mice, the Zika virus did not significantly affect the development of lethal infection caused by WNV. At the same time, prior infection of mice with non-lethal doses of the Zika virus 4 and 20 days before the administration of WNV provided effective protection for the animals against lethal WNV infection and the emergence of a high level of virus-neutralizing antibodies against this virus.
About the authors
Victor A. Svyatchenko
State Research Center of Virology and Biotechnology «Vector»
Email: svyat@vector.nsc.ru
ORCID iD: 0000-0002-2729-0592
Candidate of Biological Sciences, Leading Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionElena V. Protopopova
State Research Center of Virology and Biotechnology «Vector»
Email: protopopova_ev@vector.nsc.ru
ORCID iD: 0000-0002-2782-8364
Candidate of Biological Sciences, Leading Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionStanislav S. Legostaev
State Research Center of Virology and Biotechnology «Vector»
Email: legostaev_ss@vector.nsc.ru
ORCID iD: 0000-0002-6202-445X
junior researcher of the department of molecular virology of flaviviruses and viral hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionTamara P. Mikryukova
State Research Center of Virology and Biotechnology «Vector»
Email: mikryukova_tp@vector.nsc.ru
ORCID iD: 0000-0003-4350-4260
Candidate of Biological Sciences, Senior Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionAlexander P. Agafonov
State Research Center of Virology and Biotechnology «Vector»
Email: agafonov@vector.nsc.ru
ORCID iD: 0000-0003-2577-0434
Doctor of Biological Sciences, Director General
Russian Federation, 630559, Koltsovo, Novosibirsk RegionValery B. Loktev
State Research Center of Virology and Biotechnology «Vector»
Author for correspondence.
Email: loktev@vector.nsc.ru
ORCID iD: 0000-0002-0229-321X
MD, PhD, DSc, Prof., academician RANS, Chief Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionReferences
- Postler T.S., Beer M., Blitvich B.J., Bukh J., de Lamballerie X., Drexler J.F., et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023; 168(9): 224. https://doi.org/10.1007/s00705-023-05835-1
- Baker R.E., Mahmud A.S., Miller I.F., Rajeev M., Rasambainarivo F., Rice B.L., et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022; 20(4): 193–05. https://doi.org/10.1038/s41579-021-00639-z
- Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013; 310(3): 308–15. https://doi.org/10.1001/jama.2013.8042
- van Leur S.W., Heunis T., Munnur D., Sanyal S. Pathogenesis and virulence of flavivirus infections. Virulence. 2021; 12(1): 2814–38. https://doi.org/10.1080/21505594.2021.1996059
- Brüssow H., Figuerola J. The spread of the mosquito-transmitted West Nile virus in North America and Europe. Microb. Biotechnol. 2025; 18(3): e70120. https://doi.org/10.1111/1751-7915.70120
- Kariwa H., Murata R., Totani M., Yoshii K., Takashima I. Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia. Int. J. Environ. Res. Public Health. 2013; 10(12): 7144–64. https://doi.org/10.3390/ijerph10127144
- Korobitsyn I.G., Moskvitina N.S., Tyutenkov O.Y., Gashkov S.I., Kononova Y.V., Moskvitin S.S., et al. Detection of tick-borne pathogens in wild birds and their ticks in Western Siberia and high level of their mismatch. Folia Parasitol. (Praha). 2021; 68: 2021.024. https://doi.org/10.14411/fp.2021.024
- Duggal N.K., Langwig K.E., Ebel G.D., Brault A.C. On the fly: interactions between birds, mosquitoes, and environment that have molded West Nile virus genomic structure over two decades. J. Med. Entomol. 2019; 56(6): 1467–74. https://doi.org/10.1093/jme/tjz112
- Dick G.W.A., Kitchen S.F., Haddow A.J., Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952; 46: 509–20. https://doi.org/10.1016/0035-9203(52)90042-4
- Rabe I.B., Hills S.L., Haussig J.M., Walker A.T., Dos Santos T., San Martin J.L., et al. A review of the recent epidemiology of Zika virus infection. Am. J. Trop. Med. Hyg. 2025; 112(5): 1026–35. https://doi.org/10.4269/ajtmh.24-0420
- Pielnaa P., Al-Saadawe M., Saro A., Dama M.F., Zhou M., Huang Y., et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020; 543: 34–42. https://doi.org/10.1016/j.virol.2020.01.015
- Rückert C., Weger-Lucarelli J., Garcia-Luna S.M., Young M.C., Byas A.D., Murrieta R.A., et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017; 8: 15412. https://doi.org/10.1038/ncomms15412
- Laredo-Tiscareño S.V., Machain-Williams C., Rodríguez-Pérez M.A., Garza-Hernandez J.A., Doria-Cobos G.L., Cetina-Trejo R.C., et al. Arbovirus surveillance near the Mexico–us border: Isolation and sequence analysis of chikungunya virus from patients with dengue-like symptoms in Reynosa, Tamaulipas. Am. J. Trop. Med. Hyg. 2018; 99(1): 191–94. https://doi.org/10.4269/ajtmh.18-0117
- Cherabuddi K., Iovine N.M., Shah K., White S.K., Paisie T., Salemi M., et al. Zika and Chikungunya virus co-infection in a traveller returning from Colombia, 2016: virus isolation and genetic analysis. JMM Case Rep. 2016; 3(6): e005072. https://doi.org/10.1099/jmmcr.0.005072
- Iovine N.M., Lednicky J., Cherabuddi K., Crooke H., White S.K., Loeb J.C., et al. Coinfection with Zika and Dengue-2 viruses in a traveler returning from Haiti, 2016: clinical presentation and genetic analysis. Clin. Infect. Dis. 2017; 64(1): 72–5. https://doi.org/10.1093/cid/ciw667
- Laredo-Tiscareño S.V., Garza-Hernandez J.A., Salazar M.I., De Luna-Santillana E.J., Tangudu C.S., Cetina-Trejo R.C., et al. Surveillance for flaviviruses near the Mexico-U.S. border: co-circulation of Dengue virus serotypes 1, 2, and 3 and West Nile virus in Tamaulipas, Northern Mexico, 2014–2016. Am. J. Trop. Med. Hyg. 2018; 99(5): 1308–17. https://doi.org/10.4269/ajtmh.18-0426
- Slavov S.N., Gonzaga F.A.C., Pimentel B.M.S., Ramos D.D.A.R., de Araújo W.N., Covas D.T., et al. Zika virus RNA surveillance in blood donors in the Federal District of Brazil during the 2016 outbreak. Hematol. Transfus. Cell Ther. 2020; 42(4): 394–96. https://doi.org/10.1016/j.htct.2019.08.006
- Vasilakis N., Shell E.J., Fokam E.B., Mason P.W., Hanley K.A., Estes D.M., et al. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology. 2007; 358(2): 402–12. https://doi.org/10.1016/j.virol.2006.08.049
- Aaskov J., Buzacott K., Thu H.M., Lowry K., Holmes E.C. Long-term transmission of defective RNA viruses in humans and Aedes mosquitoes. Science. 2006; 311(5758): 236–38. https://doi.org/10.1126/science.1115030
- Paz-Bailey G., Adams L.E., Deen J., Anderson K.B., Katzelnick L.C. Dengue. Lancet. 2024; 403(10427): 667–82. https://doi.org/10.1016/S0140-6736(23)02576-X
- Rodriguez-Morales A.J., Villamil-Gómez W.E., Franco-Paredes C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med. Infect. Dis. 2016; 14(3): 177–9. https://doi.org/10.1016/j.tmaid.2016.05.004
- Svyatchenko V.A., Nikonov S.D., Mayorov A.P., Gelfond M.L., Loktev V.B. Antiviral photodynamic therapy: inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021; 33: 102112. https://doi.org/10.1016/j.pdpdt.2020.102112
- Toth K., Spencer J.F., Dhar D., Sagartz J.E., Buller R.M., Painter G.R., et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. USA. 2008; 105(20): 7293–97. https://doi.org/10.1073/pnas.0800200105
- Svyatchenko V.A., Ternovoi V.A., Lutkovskiy R.Y., Protopopova E.V., Gudymo A.S., Danilchenko N.V., et al. Human adenovirus and influenza A virus exacerbate SARS-CoV-2 infection in animal models. Microorganisms. 2023; 11(1): 180. https://doi.org/10.3390/microorganisms11010180
- Sanders R., Mason D.J., Foy C.A., Huggett J.F. Evaluation of digital PCR for absolute RNA quantification. PLoS One. 2013; 8(9): e75296. https://doi.org/10.1371/journal.pone.0075296
- Petrović T., Blazquez A.B., Lupulović D., Lazić G., Escribano-Romero E., Fabijan D., et al. Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: first isolation and characterisation of WNV strains from Serbia. Euro. Surveill. 2013; 18(44): 20622. https://doi.org/10.2807/1560-7917.es2013.18.44.20622
- DaPalma T., Doonan B.P., Trager N.M., Kasman L.M. A systematic approach to virus-virus interactions. Virus Res. 2010; 149(1): 1–9. https://doi.org/10.1016/j.virusres.2010.01.002
- Zhu Y., He Z., Qi Z. Virus-host Interactions in early Japanese encephalitis virus infection. Virus Res. 2023; 331: 199120. https://doi.org/10.1016/j.virusres.2023.199120
- Du Y., Wang C., Zhang Y. Viral coinfections. Viruses. 2022; 14(12): 2645. https://doi.org/10.3390/v14122645
- Gallichotte E.N., Fitzmeyer E.A., Williams L., Spangler M.C., Bosco-Lauth A.M., Ebel G.D. WNV and SLEV coinfection in avian and mosquito hosts: impact on viremia, antibody responses, and vector competence. J. Virol. 2025; 98(10): e0104124. https://doi.org/10.1128/jvi.01041-24
- Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T., et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016; 17(9): 1102–08. https://doi.org/10.1038/ni.3515
- Stettler K., Beltramello M., Espinosa D.A., Graham V., Cassotta A., Bianchi S., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016; 353(6301): 823–26. https://doi.org/10.1126/science.aaf8505
- Lobigs M., Diamond M.S. Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex. Expert Rev. Vaccines. 2012; 11(2): 177–87. https://doi.org/10.1586/erv.11.180
- Sapparapu G., Fernandez E., Kose N., Bin C., Fox J.M., Bombardi R.G., et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016; 540(7633): 443–7. https://doi.org/10.1038/nature20564
- Garg H., Yeh R., Watts D.M., Mehmetoglu-Gurbuz T., Resendes R., Parsons B., et al. Enhancement of Zika virus infection by antibodies from West Nile virus seropositive individuals with no history of clinical infection. BMC Immunol. 2021; 22(1): 5. https://doi.org/10.1186/s12865-020-00389-2
Supplementary files