Genetic diversity of Vif protein in human immunodeficiency virus type 1 variants (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1) that circulated in the Moscow region in 2019–2020
- Authors: Antonova A.A.1, Protasova L.A.1, Kim K.V.1, Munchak I.M.1, Mezhenskaya E.N.1, Orlova-Morozova E.A.2, Pronin A.Y.2, Prilipov A.G.1, Kuznetsova A.I.1
-
Affiliations:
- D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- Center for the Prevention and Control of AIDS and Infectious Diseases
- Issue: Vol 70, No 2 (2025)
- Pages: 117-132
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16744
- DOI: https://doi.org/10.36233/0507-4088-281
- EDN: https://elibrary.ru/qoqqce
- ID: 16744
Cite item
Abstract
Introduction. The Vif protein counteracts cellular deaminases, APOBEC3, which prevent viral replication. Vif is used for development of therapeutic agents. Natural polymorphisms in Vif can affect its functionality and may be associated with accelerated progression of HIV-infection to the AIDS. The study of Vif features in HIV-1 variants circulating in Russia has not been conducted previously.
The aim of the study: to study the genetic diversity of Vif in the HIV-1 variants that circulated in the Moscow region in 2019–2020.
Materials and methods. 234 whole blood samples obtained from HIV-infected patients without experience of therapy were analyzed. The study design included the following stages: extraction of proviral DNA, amplification of the vif gene, sequencing, identification of genetic variants, followed by a study of consensus sequences of the most common genetic variants of HIV-1, analysis of the conservation and genetic diversity of Vif-A6 (Vif protein of HIV-1 sub-subtype A6 variants) in patients with different stages of the disease, and assessment of genetic diversity of Vif-A6 in the Moscow region.
Results. A high degree of genetic diversity of vif gene was revealed. Consensus sequences of Vif in B and CRF63_02A6 variants were obtained for the first time. Characteristic substitutions in the consensus sequences were determined for the most common HIV-1 variants.
Conclusion. The limitation of this study is the small sample of B and CRF63_02A6. The results obtained may be of interest and may be taken into account in the development of therapeutic agents based on the Vif protein, as well as in the study of the pathogenicity of HIV-1 sub-subtype A6.
Keywords
Full Text
Introduction
The Vif protein of human immunodeficiency virus type 1 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1, HIV-1) is a viral infectivity factor that specifically antagonizes cellular deaminases of the APOBEC3, or A3 family [1, 2]. APOBEC3 family members are effectors of innate immunity that counteract many exogenous viruses, including HIV-1. Moreover, the human genome encodes 7 A3 genes (A3A, B, C, D, F, G and H) [2]. A3G (APOBEC3G) is the most potent inhibitor of HIV-1 replication in the absence of Vif protein [2, 3]. Through its cytidine deaminase activity, A3G converts cytidine (C) to uridine (U) in single-stranded non-coding viral DNA produced during reverse transcription of viral RNA. During the synthesis step of the second, coding, strand of proviral DNA, guanine (G) is replaced by adenine (A), which ultimately leads to disruption of protein synthesis and interruption of HIV-1 replication [2, 4]. Other proteins of the APOBEC3 family: A3D, A3F and A3H, exhibit different degrees of antiviral activity [5, 6]. At the same time, A3 family proteins can also exhibit antiviral activity in a deaminase-independent manner at different stages of the virus replication cycle (reverse transcription, integration, maturation) [2, 7, 8]. Vif neutralizes A3 family proteins through the attachment of these proteins to the E3 ubiquitin ligase complex, which includes Cullin 5, Elongin C, Elongin B, and CBF-β proteins, for poly-ubiquitinylation followed by proteasomal degradation. In addition, Vif is able to inhibit A3G transcription via transcriptional cofactor (CBF-β) capture, block A3G translation, and inhibit A3G incorporation into newly formed viral particles [2].
Vif protein is a highly basic polypeptide consisting of 192 amino acids, with a mass of about 23 kDa, synthesized at the late stage of virus replication [9, 10]. For many years since the discovery of the Vif protein (1986), its properties and features of interaction with host cell proteins have been studied, but its role has not been fully defined [2, 9–11]. Figure 1 shows the described functional motifs of the Vif protein.
Fig. 1. Schematic representation of functional motifs in the Vif protein. 11WQVDRMR17, 74TGERxWH80 and 171EDRW174 – sites mapping to A3F; K22, K26, Y30, 40YRHHYE45, S52, W70 and 161PPLP164 – to A3G; F39, H48 and 60GDAK63 – to A3H; 69YxxL72 – to A3G и A3F; 90RKKR93 – a signal inhibiting nuclear localisation; 108Hx5Cx17−18Cx3−5H139 (HCCH motif) – to Cullin 5, is part of the E3 ubiquitin ligase complex; 144SLQYLA149 – to Elongin C, is a member of the E3-ubiquitin ligase complex; 5WQVMIVW11, W89, T96, L106 – to CBF-β [2, 9–11].
Рис. 1. Схематическое изображение функциональных мотивов в белке Vif. 11WQVDRMR17, 74TGERxWH80 и 171EDRW174 – сайты, картирующиеся с A3F; K22, K26, Y30, 40YRHHYE45, S52, W70 и 161PPLP164 – с A3G; F39, H48 и 60GDAK63 – с A3H; 69YxxL72 – с A3G и A3F; 90RKKR93 – сигнал, ингибирующий ядерную локализацию; 108Hx5Cx17−18Cx3−5H139(HCCH мотив) – с Cullin 5, входит в состав E3-убиквитин-лигазного комплекса; 144SLQYLA149 – с Elongin C, входит в состав E3-убиквитин-лигазного комплекса; 5WQVMIVW11, W89, T96, L106 – с CBF-β [2, 9–11].
Earlier studies showed that natural polymorphisms in the Vif protein can cause defects in A3G- and A3F-neutralizing activity [12]. Subsequent work has demonstrated that differences in the functional activity of the Vif protein are present between different HIV-1 variants [13, 14]. For example, 12 nucleotide substitutions were found in the region of the HIV-1 genome encoding the vif gene, sub-subtype A6, widely distributed in the Russian Federation, 4 of which were nonsynonymous and resulted in amino acid substitutions of K91Q, E134N, Q136P and I159E, compared to HIV-1 HXB2 [15]. Meanwhile, another study found an association of Vif alterations with accelerated disease progression to AIDS stage, Q136P mutation was one such alteration along with a single amino acid insertion at the 61st position and A62D/N/S substitutions [16].
Studies on the genetic diversity of the Vif protein have identified amino acid pairs and single substitutions that may be associated with both reduced CD4 cell counts and high viral load (VL) [17, 18]. Thus, natural variations in the Vif protein may influence protein functionality and disease course.
Moreover, molecules that are antagonists of the Vif protein are currently being developed, which in the future may become the basis for the development of a new class of antiretroviral drugs [19–21]. The design of many multi-epitope vaccine constructs under development includes antigens contained in the Vif protein [22]. Thus, the Vif protein is an attractive target for the development of therapeutic drugs and vaccines, which makes the study of its genetic diversity even more relevant. The high genetic diversity of HIV-1 and the uneven distribution of existing virus variants in the world determine the relevance of studying Vif protein features in locally circulating virus variants [14, 18, 23–25].
In Russia, the composition of circulating HIV-1 genetic variants is unique: the dominant variant is sub-subtype A6 (82.9%), followed by subtype B (7.14%), then the rapidly spreading recombinant form CRF63_02A6 (3.59%), and about 1% is accounted for by each of the recombinant forms CRF02_AG and CRF03_AB [26]. In our earlier studies, we investigated the features of the Tat and Rev regulatory proteins, as well as the Vpu accessory protein of HIV-1 among viruses belonging to sub-subtype A6, which is the most widespread genetic variant in the Russian Federation; in the process, we compared the genetic variability of these proteins among patients with different stages of the disease [27–30]. When comparing the consensus sequences of the Vif protein in variants of sub-subtype A6 virus circulating in different regions of Russia, characteristic features that may be associated with the so-called «founder effect» were observed [31].
The aim of the study was to investigate the genetic diversity of the Vif protein of HIV-1 variants circulating in the Moscow region: identification of genetic variants, comparison of consensus sequences of the most common ones; analysis of the conservativity and genetic diversity of the Vif-A6 protein (Vif protein of HIV-1 sub-subtype A6 variants) in patients with different stages of the disease, assessment of the genetic diversity of the Vif-A6 protein in the Moscow region.
Materials and methods
Clinical whole blood samples obtained from 234 ART-naive (without a history of antiretroviral therapy (ART)) HIV-infected patients treated at the Moscow Regional Center for AIDS Prevention and Control (hereinafter referred to as the AIDS Center) were used in this study. The blood collection procedure was performed by the staff of the AIDS Center. Blood collection was performed on a one-time basis between August 2019 and July 2020 as part of the CARE project (https://www.careresearch.eu/, accessed June 29, 2024). All obtained clinical material was used with the informed consent of patients based on the approval of the Biomedical Ethics Committee of the N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia (protocol No. 16 of 08.02.2019). Identification of risk factors for infection was carried out by interviewing patients while collecting epidemiologic anamnesis. The following additional information was also recorded: sex and age of the patient, date of clinical specimen collection, stage of the disease, VL indices and immune status of the patient (CD4+-cell count).
The patients included in the study were at stages 2, 3 and 4 of the disease (Table 1). Thus, for further analysis, 3 groups of patients were formed depending on their stage of the disease.
Table 1. Characteristics of HIV-infected patients included in the study, classified by stage of HIV infection*
Таблица 1. Характеристика включенных в исследование ВИЧ-инфицированных пациентов, классифицированных по стадии ВИЧ-инфекции*
Characteristics Характеристики | Stage 2/stage of initial manifestations 2-я стадия/стадия начальных проявлений | Stage 3/subclinical stage 3-я стадия/субклиническая стадия | Stage 4/stage of secondary manifestations 4-я стадия/стадия вторичных проявлений |
Total patients, abs. Всего пациентов, абс. | 47 | 82 | 105 |
Gender, abs. | Пол, абс. | |||
male | мужской | 29 | 46 | 75 |
female | женский | 18 | 36 | 30 |
Age, median years (range) Возраст, медиана лет (диапазон) | 38 (19–62) | 38 (21–70) | 39 (24–64) |
Infection route, abs. | Путь инфицирования, абс. | |||
hetero | гетеро | 25 | 58 | 64 |
IDU | ПИН | 5 | 11 | 36 |
MSM | МСМ | 16 | 10 | 4 |
nosocomial нозокомиальный | 0 | 0 | 1 |
unknown неизвестно | 1 | 3 | 0 |
CD4, cells/μL (range) CD4, кл/мкл (диапазон) | 642.96 (290–2022) | 474.10 (110–1658) | 236.78 (8–1062) |
Viral load, log10 RNA, copies/mL (range) Вирусная нагрузка, lg РНК, копий/мл (диапазон) | 4.9 (3.4–7.0) | 4.6 (3.3–6.2) | 5.1 (3.1–6.4) |
Note. Ranges of absolute values are presented in brackets. IDU – injecting drug users; MSM – men having sex with other men.
Примечание. В скобках представлены диапазоны абсолютных значений. ПИН – потребители инъекционных наркотиков; МСМ – мужчины, имеющие секс с мужчинами.
Table 1 shows the main demographic and clinical-epidemiologic characteristics of the patients included in the study, depending on the stage of HIV infection, according to the clinical recommendations of the Ministry of Health of Russia.
Extraction of proviral DNA as part of genomic DNA from blood cells (lymphocytes) was carried out by salting [32]. At the next stage, the vif gene was amplified using nested two-round polymerase chain reaction (PCR) followed by sequencing of the amplification products. The following sequences of primers and their amplification conditions were used in this study:
- Round 1: primers; Vif1p (GCAGGTAAGAGAGAGAGAGCAAGCTGAACA) and Vif1o (GTCTCCGCTTCTTTCTTCCTGCCATAGGA), program: 1) 95 °C – 5 min; 2) 35 cycles: 95 °C – 30 s, 57 °C – 1 min, 72 °C – 1 min 20 s; 3) 72 °C – 7 min; 4) 4 °C – ∞ (storage);
- Round 2: primers; Vif2p (GCTaCTCTGGTCTGGAAAGGTGAAGGTGAAGG) and Vif2o (TACAAGGAGGAGTCTTGGGCTGAC), program: 1) 95 °C – 5 min; 2) 35 cycles: 95 °C – 30 s, 53 °C – 1 min, 72 °C – 1 min 20 s; 3) 72 °C – 7 min; 4) 4 °C – ∞ (storage).
The amplified fragment contained 948 nucleotide bases and included the vif gene. Amplification products were purified using a commercial PCR fragment purification kit – Clean S-Cap (Eurogen, Russia) according to the manufacturer’s protocol.
Nucleotide sequences were determined by the Sanger dideoxy method using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) and primers Vif2p and Vif2o according to the manufacturer’s protocol. Sequencing reaction evaluation was performed automatically in ABI Prism 3130 Genetic Analyzer (Applied Biosystems, USA).
Assembly and editing of the obtained nucleotide sequences were performed using the SeqMan II 6.1. application (DNASTAR Inc., USA). Pairwise and multiple alignments were performed using the ClustalW module integrated into the AliView software package [33]. If problematic regions were identified in the obtained aliquots, they were further aligned manually.
Preliminary determination of HIV-1 genetic variants was performed using specialized programs: COMET HIV-1 (https://comet.lih.lu/) which uses context-oriented modeling for rapid typing of HIV-1 viruses [34]; jpHMM which determines recombinant forms of the virus using a hidden Markov model [35]; RIP 3.0 (https://www.hiv.lanl.gov/content/sequence/RIP/RIP) which serves for identification of recombinant forms of the virus; the window size in the analysis was 50 n.s., which allowed detection of recombination in short-length sequences. To clarify the results obtained, phylogenetic analysis was performed by the Maximum Likelihood (ML) method using the IQ-TREE program [36]. The source of reference sequences was the database of the Los Alamos Laboratory, USA (https://www.hiv.lanl). The nucleotide substitution model was determined using the program jModelTest v. 2.1.7 based on the Akaike information criterion (AIC) [37]. The validity of the inferred phylogenies was evaluated using bootstrap test (bootstrap) and Shimodaira-Hasegawa approximate likelihood ratio test (SH-aLRT) with 1000 post-start iterations. Clusters with SH-aLRT support > 0.9 were considered to be reliable. Visualization and graphical processing of the results of phylogenetic analysis were performed in the iTOL program [38].
The most common genetic variants of HIV-1 were then selected, and consensus amino acid sequences were generated for each of them for further comparison. For this purpose, the initially obtained HIV-1 nucleotide sequences of the most common genetic variants were translated into amino acid sequences using an online translation tool available at: https://www.bioinformatics.org/sms2/translate.html. After that, a common consensus amino acid sequence was generated based on the obtained amino acid sequences for each analyzed HIV-1 genetic variant using the Simple Consensus Maker tool (https://www.hiv.lanl.gov/content/sequence/CONSENSUS/SimpCon.html). Further, the obtained consensus sequences were compared among themselves and relative to the reference strain HXB2 HIV-1 (GenBank Accession No: K03455) using the MEGA program v. 10.2.2.
At the next stage of the study, we evaluated the conservation and genetic variability of the Vif HIV-1 sub-subtype A6 protein in patients with different stages of the disease. For this purpose, the obtained amino acid sequences of Vif-A6 were grouped according to the HIV infection stage of the patient (3 groups) from whom they were obtained. Consensus sequences were then additionally generated for each group as previously described. Thus, using the Simple Consensus Maker tool (https://www.hiv.lanl.gov/content/sequence/CONSENSUS/SimpCon.html), a common consensus amino acid sequence specific to each stage of disease (2nd, 3rd, and 4th) was generated based on the amino acid sequences obtained for each stage. The level of amino acid conservation at each position within each group, between groups, and relative to the overall consensus for the sub-subtype A6 variants obtained previously for the entire group of sequences examined (see paragraph above) was then assessed. For each amino acid position, a conservation score was performed using the following scale: 100, 90–99, 76–89, 51–75, ≤ 50%. Further, statistically significant differences in the conservation of Vif protein sequences in patients with different stages of the disease were evaluated using the Nonparametric Statistics program module of Statistica 8.0 (StatSoft Inc., USA) (Fisher’s exact two-sided test with Bonferroni multiple test correction, p < 0.0033).
To assess the genetic variability of the Vif HIV-1 sub-subtype A6 protein, the amino acid sequences of Vif-A6 were also divided into groups depending on the stage of the patient’s disease (3 groups). Amino acid substitutions were then determined (using the MEGA v. 10.2.2. program) in each patient group, using the common consensus amino acid sequence obtained for Vif HIV-1 sub-subtype A6 as a reference sequence. Sites with statistically significant differences in the frequency of occurrence in patients with different stages of the disease were identified using the Nonparametric Statistics program module of Statistica 8.0 (StatSoft Inc., USA); the level of significance was considered acceptable using the χ2 criterion with Bonferroni correction (p < 0.0005).
At the final stage of the study, the genetic diversity of the Vif protein of sub-subtype A6 in a group of virus variants circulating in the Moscow region was compared with a reference group. To form the reference group, full-genome nucleotide sequences of HIV-1 sub-subtype A6 were downloaded from the Los Alamos international database (www.hiv.lanl.gov, accessed October 17, 2024). Sequences obtained from the same patient were excluded from analysis. The total number of sequences for further analysis was 166. Then, from the downloaded full genome nucleotide sequences in the MEGA v. 10.2.2 program, the vif gene sequences were excised; the resulting nucleotide sequences of the vif gene were converted to amino acid sequences as described previously. Also, based on the obtained amino acid sequences (tested and downloaded), a common consensus sequence was formed, which was used as a reference sequence. Genetic diversity was defined as the frequency of occurrence of an amino acid different from the reference sequence in each group (Vif-A6 sequences from Moscow region and Vif-A6 sequences from non-Moscow region). Using the Nonparametric Statistics program module of Statistica 8.0 (StatSoft Inc., USA), areas with statistically significant differences were identified (p < 0.0013 using the χ2 criterion with Bonferroni correction).
Results
Initial nucleotide sequence analysis revealed that two samples (0.86%, 2/234) obtained from patients with stage 3 disease, 1311000563 and 1311001125, belonged to HIV-1 unique recombinants (URFs) and were formed by HIV-1 fragments of genetic variants A6 and B. These sequences were excluded from further phylogenetic analysis.
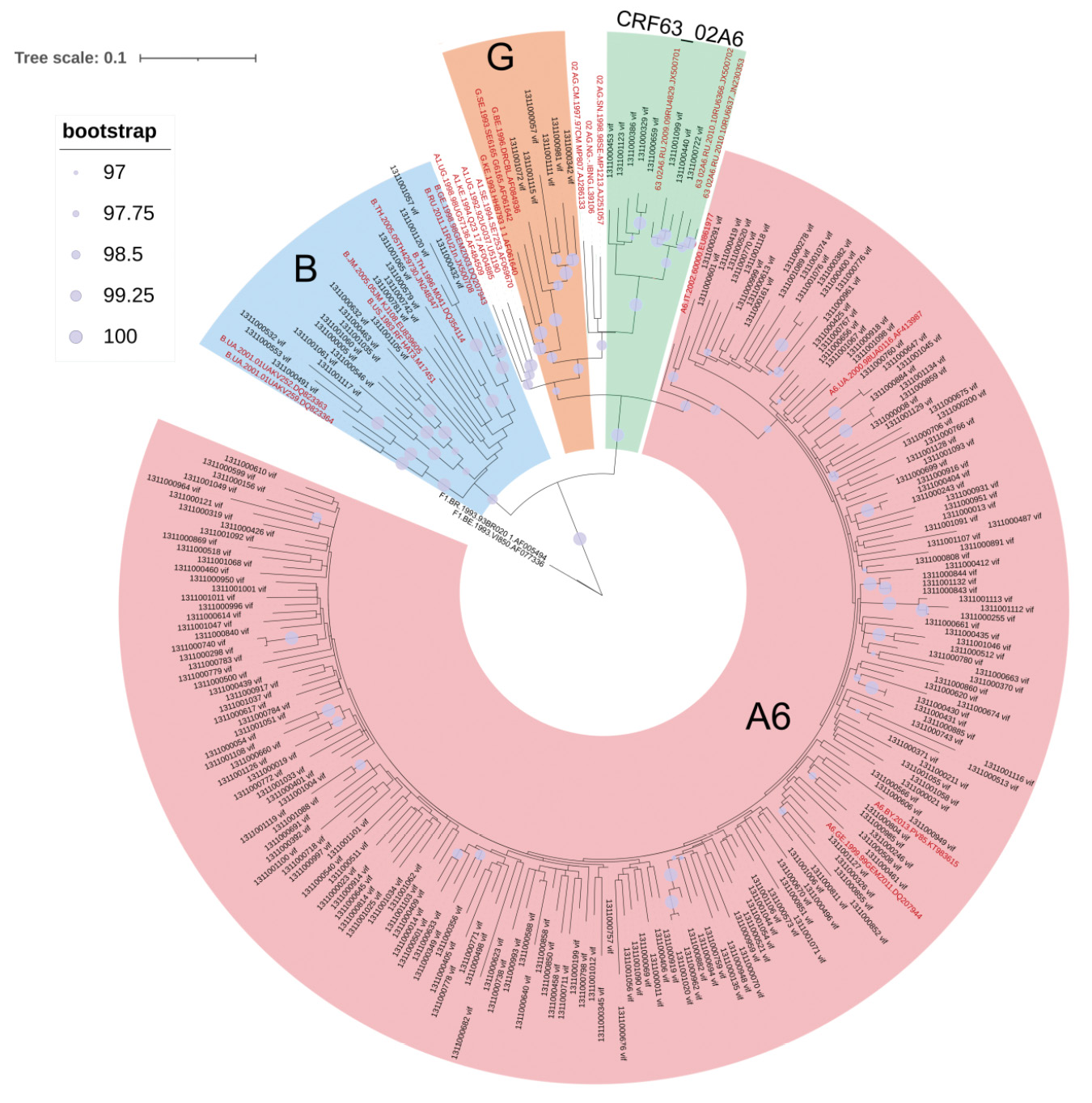
Based on the results of phylogenetic analysis, 6 (2.56%) nucleotide sequences (n.s.) were included in the cluster formed by nucleotide sequences of HIV-1 subtype G, 8 (3.42%) n.s. of circulating recombinant form CRF63_02A6 and 19 (8.12%) n.s. of subtype B. The remaining 199 (85.04%) sequences formed a significant cluster with HIV-1 sub-subtype A6 nucleotide sequences (Fig. 2).
Fig. 2. Phylogenetic analysis of nucleotide sequences of the HIV-1 vif gene (n = 257, nucleotide substitution model – GTR + I + G4). Reference sequences are highlighted in red, study sequences are highlighted in black.
Рис. 2. Филогенетический анализ нуклеотидных последовательностей гена vif ВИЧ-1 (n = 257, модель замещения нуклеотидов – GTR + I + G4). Референсные последовательности выделены красным цветом, исследуемые – черным.
The nucleotide sequences belonging to genetic variants B and G were obtained from patients with all stages of the disease, while the sequences identified as CRF63_02A6 were obtained for patients with stages 3 and 4 of the disease. It was also found that two (1311001061 and 1311001117) of the 234 investigated amino acid sequences obtained from patients with stage 2 disease had a deletion at the 131st position of the amino acid sequence. Both sequences were related to HIV-1 subtype B. One sequence (1311001065), also B subtype, obtained from a patient with stage 3 HIV infection, contained a 62insG insertion.
All nucleotide sequences of the HIV-1 vif gene (234) obtained in this study have been deposited in the GenBank international genotype database with the following numbers: PQ572780–PQ573013.
Consensus sequences of Vif protein were generated for HIV-1 variants of sub-subtype A6, subtype B, and recombinant form CRF63_02A6 as the most frequently occurring variants in the Russian Federation [26]. The total consensus amino acid sequence of Vif sub-subtype A6 was generated on the basis of 199 sequences under study: subtype B – 19, and CRF63_02A6 – 8, respectively. All generated consensus sequences consisted of 192 amino acid residues and contained neither insertions nor deletions. At the same time, in 8 amino acid sequences of sub-subtype A6 obtained from patients with stage 3 and 4 of the disease, stop codons were detected in different positions: most frequently (37.5%) in the 70th position and 25% in each of the following positions: 21, 38 and 192.
An additional evaluation of the strength of association between the studied features (presence of stop codons and HIV infection stage) using the ϕ criterion and Cramer’s V showed an insignificant strength of association.
The obtained consensus sequences were then compared. The results of the comparison of Vif HIV-1 consensus sequences of different genetic variants are presented in Figure 3.
Fig. 3. Consensus sequences of Vif HIV-1 genetic variants most frequently encountered in Russia. The dots indicate amino acid residues (a.a.r.) positions in which the a.a.r. in the consensus corresponded to the reference.
Non-polar amino acids: G (glycine), A (alanine), V (valine), L (leucine), I (isoleucine), P (proline) – are marked in blue; polar uncharged amino acids: S (serine), T (threonine), C (cysteine), M (methionine), N (asparagine), Q (glutamine) – green; aromatic amino acids: F (phenylalanine), Y (tyrosine), W (tryptophan), H (histidine) – yellow; polar acidic, negatively charged, amino acids: D (aspartic acid) and E (glutamic acid) – orange; polar basic, positively charged amino acids: K (lysine), R (arginine) – in red [39, 40].
Рис. 3. Консенсусные последовательности Vif ВИЧ-1 генетических вариантов, наиболее часто встречающихся на территории России (суб-субтипа А6, субтипа В и рекомбинантной формы CRF63) выравненные относительно референс-штамма HXB2.
Точками обозначены позиции аминокислотных остатков (АК), в которых АК в консенсусах соответствовали референсу. Аминокислоты классифицированы на основе полярности радикалов. Неполярные аминокислоты: G (глицин), A (аланин), V (валин), L (лейцин), I (изолейцин), P (пролин), отмечены синим цветом; полярные незаряженные аминокислоты: S (серин), T (треонин), C (цистеин), M (метионин), N (аспарагин), Q (глутамин) – зеленым; ароматические аминокислоты: F (фенилаланин), Y (тирозин), W (триптофан), H (гистидин) – желтым; отрицательно заряженные аминокислоты: D (аспарагиновая кислота) и E (глутаминовая кислота) – оранжевым; положительно заряженные аминокислоты: K (лизин), R (аргинин) – красным [39, 40].
When comparing the consensus sequences of HIV-1 variants circulating in the Russian Federation, differences were found in Vif HIV-1 of different genetic variants, as well as in relation to the HXB2 reference sequence.
Taking into account the absolute dominance of HIV-1 sub-subtype A6 on the territory of Russia and due to the fact that a representative sample of nucleotide sequences was obtained only for sub-subtype A6, only vif gene sequences of sub-subtype A6 were included in further analysis (a total of 199 n.s. obtained from 199 patients with different stages of the disease): 35 n.s. from patients with stage 2 (stage of initial manifestations), 68 n.s. from stage 3 (subclinical stage), and 96 from stage 4 (stage of secondary manifestations) of HIV infection.
When analyzing the conserved nature of the Vif protein in patients with different stages of the disease, it was found that the consensus sequences in each group contained predominantly amino acids similar to those in the common consensus sequence, but with different frequencies of detection (Fig. 4).
Fig. 4. Conservation of amino acid sequences of the Vif sub-subtype A6 protein in groups of patients with different stages of the disease. Amino acids are designated by a general consensus with a one-letter code: A, alanine; C, cysteine; D, aspartic acid; E, glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; M, methionine; N, asparagine; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine; W, tryptophan; Y, tyrosine.
Рис. 4. Консервативность аминокислотных последовательностей белка Vif суб-субтипа А6 в группах пациентов с разными стадиями заболевания. Аминокислоты в общем консенсусе обозначены однобуквенным кодом: А – аланин; С – цистеин; D – аспарагиновая кислота; Е – глутаминовая кислота; F – фенилаланин; G – глицин; H – гистидин; I – изолейцин; К – лизин; L – лейцин; М – метионин; Н – аспарагин; P – пролин; Q – глутамин; R – аргинин; S – серин; Т – треонин; V – валин; W – триптофан; Y – тирозин.
Evaluation of the distribution of positions with different degrees of conservation in Vif protein in different groups (by disease stage) of patients did not reveal statistically significant differences (Table 2).
Table 2. Distribution of conserved positions in the Vif protein in groups of patients with different stages of HIV infection
Таблица 2. Распределение консервативности позиций в белке Vif в группах пациентов с разными стадиями ВИЧ-инфекции
Conserved positions (%) Консервативность (%) | Number of sites (n) Число сайтов (n) | p* | ||||
stage 1 1-я стадия | stage 2 2-я стадия | stage 3 3-я стадия | p2‒3 | p3‒4 | p2‒4 | |
100 | 92 | 70 | 88 | 0.0299 | 0.0778 | 0.7591 |
90–99 | 49 | 75 | 60 | 0.0062 | 0.1344 | 0.2576 |
76–89 | 34 | 28 | 28 | 0.4883 | 1.0000 | 0.4883 |
51–75 | 15 | 5 | 12 | 0.0363 | 0.1345 | 0.6905 |
≤ 50 | 2 | 14 | 4 | 0.0036 | 0.0271 | 0.6850 |
Note. * – indicates p-value for difference between groups is indicated (Fisher’s exact two-tailed test with Bonferroni multiple test correction, p = 0.0033).
Примечание. * – указано значение p для разницы в показателе между группами (точный двусторонний тест Фишера с коррекцией множественного теста Бонферрони, p = 0,0033).
The assessment of Vif variability in patients with different stages of HIV infection revealed 35 substitutions with statistically significant differences (Table 3). However, taking into account multiple comparisons, the differences in this case become really statistically significant taking into account the Bonferroni correction, i.e. at the significance level of p < 0.0005.
Table 3. Vif-A6 amino acid substitutions with statistically significant differences in frequency of occurrence in groups of HIV-infected patients with different stages of the disease
Таблица 3. Аминокислотные замены Vif-A6 со статистически значимыми различиями по частоте встречаемости в группах ВИЧ-инфицированных пациентов с разными стадиями заболевания
Position Позиция | Mutation Мутация | stage 2 2-я стадия n = 35 | stage 3 3-я стадия n = 68 | stage 4 4-я стадия n = 96 | p* | ||
p2–3 | p2–4 | p3–4 | |||||
24 | L24I | 2 | 0 | 3 | 0.047 | – | – |
29 | M29I | 8 | 10 | 9 | – | 0.042 | – |
31 | V31I | 7 | 28 | 21 | 0.032 | – | 0.008 |
31 | V31H | 2 | 0 | 0 | 0.047 | 0.018 | – |
33 | K33G | 2 | 0 | 1 | 0.047 | – | – |
35 | A35V | 2 | 0 | 0 | 0.047 | 0.018 | – |
41 | R41I | 2 | 1 | 0 | – | 0.018 | – |
48 | H48E | 0 | 8 | 4 | 0.035 | – | – |
50 | K50R | 23 | 31 | 43 | – | 0.034 | – |
51 | V51I | 4 | 2 | 11 | – | – | 0.047 |
60 | G60R | 3 | 0 | 3 | 0.014 | – | – |
60 | G60E | 2 | 0 | 4 | 0.047 | – | – |
61 | D61E | 3 | 18 | 12 | 0.033 | – | 0.023 |
61 | D61G | 2 | 0 | 0 | 0.047 | 0.018 | – |
63 | R63M | 2 | 0 | 2 | 0.047 | – | – |
65 | V65I | 6 | 14 | 5 | – | 0.029 | 0.002 |
77 | K77R | 2 | 16 | 10 | 0.024 | – | 0.024 |
122 | K122Q | 2 | 0 | 1 | 0.047 | – | – |
122 | K122R | 0 | 5 | 1 | – | – | 0.034 |
127 | H127Q | 17 | 20 | 22 | – | 0.004 | – |
130 | S130R | 2 | 11 | 3 | – | – | 0.003 |
131 | P131H | 1 | 3 | 0 | – | – | 0.038 |
132 | R132S | 1 | 11 | 7 | 0.046 | – | – |
136 | P136S | 6 | 18 | 13 | – | – | 0.037 |
137 | A137E | 2 | 7 | 1 | – | – | 0.007 |
141 | K141Q | 4 | 3 | 15 | – | – | 0.024 |
156 | P156L | 2 | 1 | 0 | – | 0.018 | – |
157 | T157A | 3 | 1 | 1 | – | 0.027 | – |
158 | R158K | 8 | 5 | 10 | 0.025 | – | – |
159 | E159A | 2 | 0 | 0 | 0.047 | 0.018 | – |
173 | R173K | 2 | 1 | 0 | – | 0.018 | – |
181 | R181K | 1 | 9 | 15 | – | 0.048 | – |
182 | G182D | 0 | 7 | 7 | 0.049 | – | – |
184 | R184K | 4 | 1 | 4 | 0.026 | – | – |
186 | N186H | 2 | 0 | 0 | 0.047 | 0.018 | – |
Note. The p-values are presented for items with p < 0.05; items with p ≥ 0.05 are marked with ‘–’. Differences with p-value with Bonferroni correction (p < 0.0005) were considered significantly significant. * – indicates p-value for difference between groups is indicated Substitutions in functionally significant areas are marked in bold (Figure 1).
Примечание. Значения p-value представлены для всех позиций с p < 0,05; позиции с p ≥ 0,05 отмечены знаком «–». Достоверно значимыми считали различия с p с поправкой Бонферрони (p < 0,0005). * – указано значение p для разницы в показателе между группами. Жирным шрифтом отмечены замены в функционально значимых областях (рис. 1).
Thus, taking into account the Bonferroni correction, no sites with statistically significant differences in the frequency of occurrence in patients with different stages of the disease were identified.
Comparison of the genetic diversity of the Vif HIV-1 sub-subtype A6 protein in the group of virus variants circulating in the Moscow region and in the reference group revealed 39 amino acid substitutions with a difference in the frequency of occurrence (p < 0.05) (Table 4).
Table 4. Comparing genetic diversity of Vif-A6 HIV-1 between the group of virus variants circulating in the Moscow region and the reference group
Таблица 4. Сравнение генетического разнообразия Vif-A6 ВИЧ-1 в группе вариантов вируса, циркулирующих в Московской области, и референсной группе
Position Позиция | Mutation Мутация | Moscow region Московская область (n = 199) | Reference group Референсная группа (n = 166) | p |
9 | I9V | 10 | 2 | 0.042 |
17 | R17X | 1 | 9 | 0.004 |
19 | R19K | 21 | 6 | 0.012 |
20 | T20X | 1 | 18 | 0.000009 |
27 | H27Y | 19 | 6 | 0.023 |
31 | V31N | 5 | 0 | 0.04 |
31 | V31X | 7 | 16 | 0.017 |
33 | K33R | 17 | 5 | 0.027 |
41 | R41X | 2 | 10 | 0.007 |
45 | E45D | 13 | 3 | 0.028 |
51 | V51G | 0 | 4 | 0.028 |
56 | H56X | 0 | 4 | 0.028 |
61 | D61X | 2 | 7 | 0.049 |
65 | V65X | 2 | 11 | 0.004 |
67 | K67I | 9 | 1 | 0.022 |
73 | H73Q | 17 | 36 | 0.0004 |
74 | T74X | 1 | 13 | 0.0003 |
77 | K77R | 28 | 12 | 0.037 |
78 | D78A | 8 | 1 | 0.036 |
93 | R93K | 21 | 5 | 0.005 |
93 | R93M | 0 | 5 | 0.014 |
93 | R93X | 0 | 11 | 0.0002 |
98 | I98X | 5 | 12 | 0.033 |
113 | D113N | 5 | 0 | 0.04 |
128 | I128X | 0 | 5 | 0.014 |
140 | N140X | 2 | 11 | 0.004 |
141 | K141X | 0 | 5 | 0.014 |
153 | L153I | 1 | 14 | 0.0001 |
155 | T155X | 2 | 9 | 0.014 |
157 | T157A | 5 | 0 | 0.04 |
158 | R158K | 23 | 8 | 0.021 |
158 | R158X | 1 | 7 | 0.016 |
160 | R160X | 0 | 5 | 0.014 |
167 | R167X | 2 | 10 | 0.007 |
176 | K176X | 0 | 4 | 0.028 |
181 | R181X | 2 | 9 | 0.014 |
185 | G185X | 0 | 6 | 0.007 |
188 | T188S | 6 | 0 | 0.024 |
192 | C192R | 8 | 1 | 0.036 |
Note. X – amino acid is not defined. p-values are given for the positions with p < 0.05. In bold: significant in the χ2 test with Bonferroni correction p < 0.0013.
Примечание. X – аминокислота не определена. Значения p представлены для позиций с p < 0,05. Достоверно значимыми считали различия p с поправкой Бонферрони (p < 0,0013) – выделены жирным шрифтом.
With the Bonferroni correction (p < 0.0013), the frequency of occurrence of substitutions in the 5 sites differed significantly between groups.
Discussion
The application of modern antiretroviral therapy for the treatment of HIV infection prevents clinical progression of the disease, but does not eliminate viral reservoirs, i.e. does not eliminate the virus, which determines the necessity for lifelong antiretroviral drugs. Continuous use of antiretroviral drugs, in turn, over time may lead to the emergence of side effects of treatment, the formation of drug resistance of the virus and the problem of drug-drug interactions. In this regard, the world is constantly working on the development of new antiretroviral drugs, and considerable efforts are being made to create a means of complete cure for HIV infection. As part of this work, Vif protein antagonists with different principles of action are also being designed: inhibitors of Vif protein multimerization, inhibitors of direct binding of Vif to A3G, inhibitors of Vif binding to Elongin C, and inhibitors of Vif binding to CBF-β. [19, 41]. Furthermore, the Vif protein is being used as a basis for the development of therapeutic vaccines and gene therapy of HIV infection. Thus, in vitro experiments demonstrated that a multi-epitope construct containing nonstructural Vif protein had a high efficiency of cell penetration [42]. Lentiviral vectors for gene therapy of HIV infection encoding A3Gs resistant to the action of Vif protein are being developed [43]. In the present study, the features of the Vif protein were studied for the first time in HIV-1 variants circulating in Russia.
The results of the distribution of HIV-1 genetic variants in the present study and in an earlier large-scale study of HIV-1 genetic diversity in the Russian Federation correlate well with each other: sub-subtype A6 – 85.04%, subtype B – 8.12%, CRF63_02A6 – 3.42% and sub-subtype A6 – 82.9%, subtype B – 7.14%, CRF63_02A6 – 3.59%, respectively. However, in the present study, 2.56% of the sequences were genotyped as subtype G, whereas in a larger study, subtype G accounted for less than 1% of the samples analyzed [26]. This discrepancy may be explained by the fact that some of the sequences that were identified as subtype G in the present study for the vif gene may in fact be circulating recombinant forms or unique recombinants. For example, certain HIV-1 variants initially genotyped by the pol gene as subtype G were subsequently identified by full-genome sequencing as the recombinant form BG [44].
Based on the results of this study, it was found that two sequences obtained from patients with stage 2 HIV infection contained deletions; one sequence obtained from a patient with stage 3 HIV infection contained an insertion (62insG). In 8 sequences obtained from patients with stage 3 (3 sequences) and stage 4 (5 sequences) disease, stop codons were detected in different positions. Most often (in 37.5% of amino acid sequences containing stop codons), the stop codons were in the 70th position. In 25% of the sequences, stop codons were found in each of the following positions: 21, 38, and 192. Considering the high frequency of stop codons in certain positions, we can make an assumption about a non-random mechanism of this phenomenon, which is related to the action of cellular protein A3G [2, 4].
The strength of the relationship between the presence of stop codons and the stage of the disease was not significant. At the same time, a number of studies have noted that the amount of defective provirus increases during the course of HIV infection, and its share can account for about 90% of the entire HIV reservoir [45, 46].
However, the existing limitations of the study, such as the relatively small sample size and the lack of availability of sequences from the same patient in the dynamics, as well as individual peculiarities of the course of the disease in each particular person, do not allow us to speak about any trends regarding stop codons in Vif sequences. The possibility of premature stop codons with preservation of protein functional activity has been repeatedly noted for Rev and Tat proteins, whereas no such data are available for Vif protein to date, so it is not possible to assess the functional significance of each stop codon position either [47, 48].
When comparing the consensus sequences of HIV-1 variants circulating in Russia, it was found that the consensus sequence of the Vif protein of sub-subtype A6 differed from the HXB2 reference sequence in 30 positions, subtype B – in 16 positions, recombinant form CRF63_02A6 – in 32 positions. The highest number of substitutions, 2 or more, of the Vif protein consensus of HIV-1 variants circulating in Russia relative to HXB2 contained in positions: 47, 128, 134, 151, 159 и 167. The Vif protein consensus of HIV-1 variants circulating in Russia also differed among themselves: the consensus of sub-subtype A6 differed from the consensus of sub-subtype A6 in 28 out of 192 positions (by 14.6%), and the consensus of the recombinant form CRF63_02A6 – in 25 out of 192 positions (by 13%). Furthermore, it should be noted that the circulating recombinant form CRF63_02A6 is a secondary recombinant formed by HIV-1 sub-subtype A6 and CRF02_A6G, and the vif gene is a recombination region with inclusion of fragments of both virus variants (https://www.hiv.lanl.gov/components/sequence/HIV/crfdb/crfs.comp).
It is important to note that consensus variants of HIV-1 circulating in Russia differed from the reference strain HXB2 in 7 functionally significant positions and contained the following substitutions: K22N, F39V, R63K, T74K, R77K, K91Q, K92E (Fig. 1). Substitutions of arginine (R) for lysine (K) in the 63rd and 77th positions do not lead to a change in the type of amino acid in the analyzed position, i.e. the amino acid remains positively charged, whereas substitutions of K22N, F39V, T74K, K91Q, K92E lead to a change in the type of amino acid (Fig. 1), which may affect the change in the functional properties of the protein. For example, it was previously shown that the presence of a positive charge in a functionally significant motif, which includes K22, is important for A3G binding and inactivation [49]. Thus, replacing lysine (K), a positively charged amino acid at position 22, with asparagine (N), a polar uncharged amino acid, may result in a decrease in positive charge, which in turn may affect A3G binding and inactivation.
Evaluation of the distribution of position conservation in the Vif protein did not reveal a significant difference between the groups of patients with different stages of the disease (Table 3), which is consistent with the results of similar studies for the Vpu protein [29].
The results of the study of Vif variability in patients with different stages of HIV infection did not reveal substitutions with statistically significant differences in their frequency of occurrence (Table 3), which distinguishes the results of this study from the results of the study of other nonstructural proteins, Tat and Vpu [28, 29]. The analysis of substitutions in the functionally significant regions of Vif protein (Table 3) in patients with different stages of the disease also does not allow us to reveal any trend in their frequency of occurrence. In an earlier study of the genetic diversity of the Vif protein of HIV-1 subtype B, it was shown that patients infected with virus variants containing the K22H substitution tended to have lower CD4-cell counts and higher VL [18]. In the present study, no difference in the incidence (p < 0.05) of the K22H substitution was found in patients with different stages of the disease.
No characteristic substitutions significantly more frequent in HIV-1 sub-subtype A6 variants circulating in the Moscow region were found, while the frequency of occurrence of substitutions in 5 sites (T20X, H73Q, T74X, R93X, L153I) was significantly higher in the reference group (Table 4). A similar study of the genetic diversity of Vif subtype C protein in Uganda, South Africa, and India revealed specific amino acid substitutions characteristic of the geographical regions under study [50]. The results we obtained may be related to the specificity of the region under study. Thus, the Moscow metropolitan region (including Moscow and the Moscow region) is the largest transportation hub in Russia, which causes the intersection of large migration flows, in turn leading to the constant circulation and change of HIV-1 sub-subtype A6 variants from different regions of our country, as well as the countries of the former Soviet Union. All this may prevent the formation of any pronounced features of circulating HIV-1 sub-subtype A6 variants in the Moscow region [51].
Conclusion
The study was the first to comprehensively analyze the features of the vif protein of HIV-1 variants circulating in Russia, in the Moscow region. The composition of HIV-1 genetic variants circulating in the Moscow region, as determined by the vif gene, predominantly correlated with the composition of HIV-1 genetic variants circulating in the Russian Federation as a whole. In this study, consensus sequences of HIV-1 vif protein of genetic variants B and CRF63_02A6 circulating in Russia were obtained and presented for the first time. Amino acid substitutions in functionally significant positions of the Vif protein, characteristic of HIV-1 variants most common in Russia, have been identified; these substitutions may potentially affect changes in protein properties and may be taken into account in the development of therapeutic drugs based on the Vif protein in the future. Comparison of the conservation and genetic variability of the Vif-A6 protein in patients with different stages of HIV infection did not reveal any significant differences. The positions containing deletions, insertions, and stop codons in the region of the HIV-1 genome encoding the Vif protein were identified. In general, the genetic diversity of the Vif-A6 protein of HIV-1 virus variants circulating in the Moscow region was lower than in the general population of HIV-1 sub-subtype A6 variants. This study has a limitation due to the small sample of Vif sequences of HIV-1 genetic variants – B and CRF63_02A6.
About the authors
Anastasiia A. Antonova
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: anastaseika95@mail.ru
ORCID iD: 0000-0002-9180-9846
PhD, Researcher, Laboratory of T-lymphotropic viruses
Russian Federation, 123098, MoscowLarisa A. Protasova
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: larisa.protasova.03@mail.ru
ORCID iD: 0009-0001-0430-1578
research assistant, Laboratory of T-lymphotropic viruses
Russian Federation, 123098, MoscowKristina V. Kim
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: kimsya99@gmail.com
ORCID iD: 0000-0002-4150-2280
junior researcher, Laboratory of T-lymphotropic viruses
Russian Federation, 123098, MoscowIana M. Munchak
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: yana_munchak@mail.ru
ORCID iD: 0000-0002-4792-8928
junior researcher, Laboratory of T-lymphotropic viruses
Russian Federation, 123098, MoscowEkaterina N. Mezhenskaya
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: belokopytova.01@mail.ru
ORCID iD: 0000-0002-3110-0843
PhD, Researcher, Laboratory of T-lymphotropic viruses
Russian Federation, 123098, MoscowElena A. Orlova-Morozova
Center for the Prevention and Control of AIDS and Infectious Diseases
Email: orlovamorozova@gmail.com
ORCID iD: 0000-0003-2495-6501
PhD, Head of outpatient department
Russian Federation, 140053, Moscow region, KotelnikiAlexander Yu. Pronin
Center for the Prevention and Control of AIDS and Infectious Diseases
Email: alexanderp909@gmail.com
ORCID iD: 0000-0001-9268-4929
PhD, Chief Physician
Russian Federation, 140053, Moscow region, KotelnikiAlexey G. Prilipov
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: a_prilipov@mail.ru
ORCID iD: 0000-0001-8755-1419
Doctor of Biological Sciences, leading researcher, head of the laboratory of molecular genetics
Russian Federation, 123098, MoscowAnna I. Kuznetsova
D.I. Ivanovsky Institute of Virology of National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: a-myznikova@list.ru
ORCID iD: 0000-0001-5299-3081
Head of laboratory of T-lymphotropic viruses, PhD, leading researcher
Russian Federation, 123098, MoscowReferences
- Rose K.M., Marin M., Kozak S.L., Kabat D. The viral infectivity factor (Vif) of HIV-1 unveiled. Trends Mol. Med. 2004; 10(6): 291–7. https://doi.org/10.1016/j.molmed.2004.04.008
- Stupfler B., Verriez C., Gallois-Montbrun S., Marquet R., Paillart J.C. Degradation Independent inhibition of APOBEC3G by the HIV-1 Vif protein. Viruses. 2021; 13(4): 617. https://doi.org/10.3390/v13040617
- Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002; 418(6898): 646–50. https://doi.org/10.1038/nature00939
- Mangeat B., Turelli P., Caron G., Friedli M., Perrin L., Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003; 424(6944): 99–103. https://doi.org/10.1038/nature01709
- Hultquist J.F., Lengyel J.A., Refsland E.W., LaRue R.S., Lackey L., Brown W.L., et al. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-Deficient HIV-1. J. Virol. 2011; 85(21): 11220–34. https://doi.org/10.1128/JVI.05238-11
- Wang X., Abudu A., Son S., Dang Y., Venta P.J., Zheng Y.H. Analysis of human APOBEC3H haplotypes and anti-human immunodeficiency virus type 1 activity. J. Virol. 2011; 85(7): 3142–52. https://doi.org/10.1128/JVI.02049-10
- Guo F., Cen S., Niu M., Yang Y., Gorelick R.J., Kleiman L. The interaction of APOBEC3G with human immunodeficiency virus type 1 nucleocapsid inhibits tRNA3Lys annealing to viral RNA. J. Virol. 2007;81(20):11322–31. https://doi.org/10.1128/JVI.00162-07
- Xu W.K., Byun H., Dudley J.P. The role of APOBECs in viral replication. Microorganisms. 2020; 8(12): 1899. https://doi.org/10.3390/microorganisms8121899
- Azimi F.C., Lee J.E. Structural perspectives on HIV-1 Vif and APOBEC3 restriction factor interactions. Protein Sci. 2020; 29(2): 391–406. https://doi.org/10.1002/pro.3729
- Friedler A., Zakai N., Karni O., Friedler D., Gilon C., Loyter A. Identification of a nuclear transport inhibitory signal (NTIS) in the basic domain of HIV-1 Vif protein. J. Mol. Biol. 1999; 289(3): 431–7. https://doi.org/10.1006/jmbi.1999.2785
- Takaori-Kondo A., Shindo K. HIV-1 Vif: a guardian of the virus that opens up a new era in the research field of restriction factors. Front. Microbiol. 2013; 4: 34. https://doi.org/10.3389/fmicb.2013.00034
- Simon V., Zennou V., Murray D., Huang Y., Ho D.D., Bieniasz P.D. Natural variation in Vif: differential impact on APOBEC3G/3F and a potential role in HIV-1 diversification. PLoS Pathog. 2005; 1(1): e6. https://doi.org/10.1371/journal.ppat.0010006
- Iwabu Y., Kinomoto M., Tatsumi M., Fujita H., Shimura M., Tanaka Y., et al. Differential anti-APOBEC3G activity of HIV-1 Vif proteins derived from different subtypes. J. Biol. Chem. 2010; 285(46): 35350–8. https://doi.org/10.1074/jbc.M110.173286
- Ronsard L., Raja R., Panwar V., Saini S., Mohankumar K., Sridharan S., et al. Genetic and functional characterization of HIV-1 Vif on APOBEC3G degradation: First report of emergence of B/C recombinants from North India. Sci. Rep. 2015; 5: 15438. https://doi.org/10.1038/srep15438
- Gromov K.B., Laga V.Y., Murzakova A.V., Kireev D.E. Analysis of polymorphism of non-structural HIV-1 Vif and Rev genes. In: Molecular Diagnostics – 2017: Proceedings of the IX All-Russian Scientific and Practical Conference with International Participation [Molekulyarnaya diagnostika – 2017: sbornik trudov IKH Vserossiiskoi nauchno-prakticheskoi konferentsii s mezhdunarodnym uchastiem]. Moscow; 2017: 455–6. (in Russian)
- De Maio F.A., Rocco C.A., Aulicino P.C., Bologna R., Mangano A., Sen L. Effect of HIV-1 Vif variability on progression to pediatric AIDS and its association with APOBEC3G and CUL5 polymorphisms. Infect. Genet. Evol. 2011; 11(6): 1256–62. https://doi.org/10.1016/j.meegid.2011.04.020
- Bizinoto M.C., Yabe S., Leal É., Kishino H., Martins L. de O., de Lima M.L., et al. Codon pairs of the HIV-1 vif gene correlate with CD4+ T cell count. BMC Infect. Dis. 2013; 13: 173. https://doi.org/10.1186/1471-2334-13-173
- Villanova F., Barreiros M., Janini L.M., Diaz R.S., Leal É. Genetic diversity of HIV-1 gene Vif among treatment-naive Brazilians. AIDS Res. Hum. Retroviruses. 2017; 33(9): 952–9. https://doi.org/10.1089/AID.2016.0230
- Bennett R.P., Salter J.D., Smith H.C. A new class of antiretroviral enabling innate immunity by protecting APOBEC3 from HIV Vif-dependent degradation. Trends Mol. Med. 2018; 24(5): 507–20. https://doi.org/10.1016/j.molmed.2018.03.004
- Sharkey M., Sharova N., Mohammed I., Huff S.E., Kummetha I.R., Singh G., et al. HIV-1 escape from small-molecule antagonism of Vif. mBio. 2019; 10(1): e00144-19. https://doi.org/10.1128/mBio.00144-19
- Duan S., Wang S., Song Y., Gao N., Meng L., Gai Y., et al. A novel HIV-1 inhibitor that blocks viral replication and rescues APOBEC3s by interrupting Vif/CBFβ interaction. J. Biol. Chem. 2020; 295(43): 14592–605. https://doi.org/10.1074/jbc.RA120.013404
- Akbari E., Seyedinkhorasani M., Bolhassani A. Conserved multiepitope vaccine constructs: A potent HIV-1 therapeutic vaccine in clinical trials. Braz. J. Infect. Dis. 2023; 27(3): 102774. https://doi.org/10.1016/j.bjid.2023.102774
- Guerra-Palomares S.E., Hernandez-Sanchez P.G., Esparza-Perez M.A., Arguello J.R., Noyola D.E., Garcia-Sepulveda C.A. Molecular characterization of Mexican HIV-1 Vif sequences. AIDS Res. Hum. Retroviruses. 2016; 32(3): 290–5. https://doi.org/10.1089/AID.2015.0290
- Bbosa N., Kaleebu P., Ssemwanga D. HIV subtype diversity worldwide. Curr. Opin. HIV AIDS. 2019; 14(3): 153–60. https://doi.org/10.1097/COH.0000000000000534
- Williams M.E. HIV-1 Vif protein sequence variations in South African people living with HIV and their influence on Vif-APOBEC3G interaction. Eur. J. Clin. Microbiol. Infect. Dis. 2024; 43(2): 325–38. https://doi.org/10.1007/s10096-023-04728-0
- Antonova A.A., Kuznetsova A.I., Ozhmegova E.N., Lebedev A.V., Kazennova E.V., Kim K.V., et al. Genetic diversity of HIV-1 at the current stage of the epidemic in the Russian Federation: an increase in the prevalence of recombinant forms. VICH-infektsiya i immunosupressii. 2023; 15(3): 61–72. https://doi.org/10.22328/2077-9828-2023-15-3-61-72 https://elibrary.ru/tpwttn (in Russian)
- Kuznetsova A.I., Gromov K.B., Kireev D.E., Shlykova A.V., Lopatukhin A.E., Kazennova E.V., et al. Analysis of Tat protein characteristics in human immunodeficiency virus type 1 sub-subtype A6 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1). Voprosy virusologii. 2021; 66(6): 452–63. https://doi.org/10.36233/0507-4088-83 https://elibrary.ru/cmzgyc (in Russian)
- Kuznetsova A., Kim K., Tumanov A., Munchak I., Antonova A., Lebedev A., et al. Features of Tat protein in HIV-1 sub-subtype A6 variants circulating in the Moscow Region, Russia. Viruses. 2023; 15(11): 2212. https://doi.org/10.3390/v15112212 https://elibrary.ru/ucqyal
- Antonova A.A., Lebedev A.V., Kazennova E.V., Kim K.V., Ozhmegova E.N., Tumanov A.S., et al. Variability of VPU protein in HIV-1 sub-subtype A6 in patients with different stages of HIV infection. VICH-infektsiya i immunosupressii. 2024; 16(2): 40–50. https://doi.org/10.22328/2077-9828-2024-16-2-40-50 https://elibrary.ru/lpjxqk (in Russian)
- Lebedev A., Kim K., Ozhmegova E., Antonova A., Kazennova E., Tumanov A., et al. Rev protein diversity in HIV-1 group M clades. Viruses. 2024; 16(5): 759. https://doi.org/10.3390/v16050759
- Antonova A.A., Lebedev A.V., Ozhmegova E.N., Shlykova A.V., Lapavok I.A., Kuznetsova A.I. Variability of non-structural proteins in HIV-1 sub-subtype A6 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1, sub-subtype A6) variants circulating in different regions of the Russian Federation. Voprosy virusologii. 2024; 69(5): 470–80. https://doi.org/10.36233/0507-4088-262 https://elibrary.ru/wbbkuq (in Russian)
- Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic. Acids. Res. 1988; 16(3): 1215. https://doi.org/10.1093/nar/16.3.1
- Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014; 30(22): 3276–8. https://doi.org/10.1093/bioinformatics/btu531
- Struck D., Lawyer G., Ternes A.M., Schmit J.C., Bercoff D.P. COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014; 42(18): e144. https://doi.org/10.1093/nar/gku739
- Schultz A.K., Bulla I., Abdou-Chekaraou M., Gordien E., Morgenstern B., Zoaulim F., et al. jpHMM: recombination analysis in viruses with circular genomes such as the hepatitis B virus. Nucleic Acids Res. 2012; 40: W193-8. https://doi.org/10.1093/nar/gks414.
- Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015; 32(1): 268–74. https://doi.org/10.1093/molbev/msu300
- Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012; 9(8): 772. https://doi.org/10.1038/nmeth.2109.
- Letunic I., Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021; 49(W1): W293–6. https://doi.org/10.1093/nar/gkab301
- Berezov T.T., Korovkin B.F. Biological Chemistry [Biologicheskaya khimiya]. Moscow: Meditsina; 1998 (in Russian)
- Lobanov M.Y., Pereyaslavets L.B., Likhachev I.V., Matkarimov B.T., Galzitskaya O.V. Is there an advantageous arrangement of aromatic residues in proteins? Statistical analysis of aromatic interactions in globular proteins. Comput. Struct. Biotechnol. J. 2021; 19: 5960–8. https://doi.org/10.1016/j.csbj.2021.10.036
- Duan S., Wang S., Song Y., Gao N., Meng L., Gai Y., et al. A novel HIV-1 inhibitor that blocks viral replication and rescues APOBEC3s by interrupting vif/CBFβ interaction. J. Biol. Chem. 2020; 295(43): 14592–605. https://doi.org/10.1074/jbc.RA120.013404
- Kardani K., Hashemi A., Bolhassani A. Comparison of HIV-1 Vif and Vpu accessory proteins for delivery of polyepitope constructs harboring Nef, Gp160 and P24 using various cell penetrating peptides. PLoS One. 2019; 14(10): e0223844. https://doi.org/10.1371/journal.pone.0223844
- Delviks-Frankenberry K.A., Ackerman D., Timberlake N.D., Hamscher M., Nikolaitchik O.A., Hu W.S., et al. Development of Lentiviral Vectors for HIV-1 Gene Therapy with Vif-Resistant APOBEC3G. Mol. Ther. Nucleic Acids. 2019; 18: 1023–38. https://doi.org/10.1016/j.omtn.2019.10.024
- Murzakova A., Kireev D., Baryshev P., Lopatukhin A., Serova E., Shemshura A., et al. Molecular epidemiology of HIV-1 subtype G in the Russian Federation. Viruses. 2019; 11(4): 348. https://doi.org/10.3390/v11040348
- Bobkova M.R. Defective HIV proviruses: possible involvement in the HIV infection pathogenesis. Voprosy virusulogii. 2024; 69(5): 399–414. https://doi.org/10.36233/0507-4088-261 https://elibrary.ru/pselci (in Russian)
- Veselova E.I., Kaminskiy G.D., Samoylova A.G., Vasilyeva I.A. HIV reservoir in HIV patients. Tuberkulez i bolezni legkikh. 2019; 97(5): 50–7. http://doi.org/10.21292/2075-1230-2019-97-5-50-57 https://elibrary.ru/hfadpt (in Russian)
- Jayaraman B., Fernandes J.D., Yang S., Smith C., Frankel A.D. Highly mutable linker regions regulate HIV-1 rev function and stability. Sci. Rep. 2019; 9(1): 5139. https://doi.org/10.1038/s41598-019-41582-7
- Li L., Dahiya S., Kortagere S., Aiamkitsumrit B., Cunningham D., Pirrone V., et al. Impact of Tat genetic variation on HIV-1 disease. Adv. Virol. 2012; 2012: 123605. https://doi.org/10.1155/2012/123605
- Chen G., He Z., Wang T., Xu R., Yu X.F. A patch of positively charged amino acids surrounding the human immunodeficiency virus type 1 Vif SLVx4Yx9Y motif influences its interaction with APOBEC3G. J. Virol. 2009; 83(17): 8674–82. https://doi.org/10.1128/JVI.00653-09
- Williams ME. HIV-1 Vif protein sequence variations in South African people living with HIV and their influence on Vif-APOBEC3G interaction. Eur. J. Clin. Microbiol. Infect. Dis. 2024; 43(2): 325–38. https://doi.org/10.1007/s10096-023-04728-0
- Savchenko-Belsky V., Maltseva M., Maslova A. Problems and prospects of the development of the transport system of the Moscow agglomeration. Transportnoe delo Rossii. 2022; (1): 124–7. https://doi.org/10.52375/20728689_2022_1_124 https://elibrary.ru/cctqsp (in Russian)
Supplementary files