The effect of sodium deoxyribonucleate with iron complex on the expression of surface markers of MT-4 cells infected with human immunodeficiency virus type 1 (HIV-1) (Retroviridae: Primate lentivirus group)
- Authors: Nosik D.N.1, Kalnina L.B.1, Selimova L.M.1, Kaplina E.N.2
-
Affiliations:
- FSBI «National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya» of the Ministry of Health of Russia
- LLC «PharmPak»
- Issue: Vol 69, No 4 (2024)
- Pages: 309-319
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16658
- DOI: https://doi.org/10.36233/0507-4088-240
- EDN: https://elibrary.ru/uzlpzs
- ID: 16658
Cite item
Abstract
Introduction. The persistence of immune dysfunction during therapy has serious consequences for the health of HIV-infected people. Therefore, an important direction is the search for drugs that can reduce the inflammatory potential of the immune system and serve as an additional component of antiviral therapy.
Aim ‒ to study the effect of the immunomodulatory drug Sodium deoxyribonucleate with iron complex (DNA-Na-Fe) on the expression of activation markers in MT-4 cells infected with HIV-1.
Materials and methods. Expression levels of CD4, CD28, CD38, CD62L and HLA-DR proteins on the plasma membrane were measured in cells. To assess viral activity, the p24 protein was quantified by ELISA.
Results and discussion. The two cell variants with different replicative activity were analyzed. Control cells, cells with DNA-Na-Fe, infected cells and infected cells with DNA-Na-Fe were tested. Based on the results obtained, it can be concluded that antiviral activity of the drug in MT-4 cells infected with HIV-1 is associated with immunomodulatory activity that enhances the expression of membrane proteins CD4, CD28, CD38 and CD62L. Diversity in the effect of DNA-Na-Fe on the studied surface proteins expression in two cell lines indicates that they depend on the characteristics of the combined molecular biological processes occurring in cells. And the increased effects observed in a system with changes in replicative activity assumes its active participation in virus replication at the stages of virus penetration and budding.
Conclusion. Studies have shown that DNA-Na-Fe has antiviral and immunomodulatory activity.
Full Text
Introduction
The course of infection caused by human immunodeficiency virus (HIV-1) is closely linked to the functioning of the immune system. HIV-1 infects cells of the immune system containing the major marker CD4. As a result of well-coordinated pathogenic mechanisms utilized by the virus, the development of infection leads to impaired functioning of all branches of immunity. Host immune responses contribute to the control and suppression of infection through the activation of several transduction pathways that serve to neutralize viral replication mechanisms in infected cells [1]. A comprehensive study of HIV-host cell interactions is key to the development of effective treatment regimens for the infection. Additional understanding of the immune control of HIV-1 in individuals who control the infection also seems important [2]. Despite advances in prevention and treatment, a major obstacle to the efficacy of chemotherapy is the emergence of long-lived latent reservoirs of virus [3]. During more than 40 years of studying HIV infection and the development of a large group of effective antiviral drugs and the introduction of comprehensive antiretroviral therapy (ART), significant results have been achieved to improve the organization of measures to combat the spread of the disease and improve the quality of life of people living with HIV (PLHIV). According to the Joint United Nations Program on HIV/AIDS (UNAIDS), by 2023, the number of PLHIV constitutes about 39 (33.1–45.7) million people. Five countries – Botswana, Eswatini, Rwanda, Tanzania, Zimbabwe – have already met the testing and treatment targets of the new UNAIDS 2021–2026 strategy by 2022. The goal of the strategy is to reach «95–95–95» (95% of PLHIV know their HIV status, 95% who know their status are on ART, 95% who are on therapy have achieved an undetectable viral load). Despite this undoubted success, current treatment strategies have limitations. Lifelong treatment is challenging for many patients. Adherence to ART remains a challenge, especially among those patients who cannot fully adhere to treatment. Drug toxicity and the persistence of immune dysfunction on ART have serious health consequences. These factors emphasize the importance of finding new means to combat the virus. Finding effective methods of therapy is now a key priority for PLHIV. Special attention is given to the problem of studying immunomodulatory drugs that can reduce the inflammatory potential of immune system cells [4]. Targeted treatment of inflammation is a likely strategy to reduce HIV risk and slow disease progression. Chronic inflammation and impaired CD4+ T-lymphocyte function are seen even with effective ART. Approximately 15–30% of patients do not have optimal recovery of CD4+ T-cell counts [5]. Drugs based on natural raw materials with reduced side effects combined with anti-inflammatory properties are promising options. Sodium deoxyribonucleate (DNA-Na) and its complex with iron (DNA-Na-Fe), based on double-stranded DNA of natural origin, have antiviral and immunomodulatory properties [6, 7].
The aim of this study was to investigate the expression of CD4, CD28, CD38, CD62L and HLA-DR proteins of the cytoplasmic membrane of CD4+ T-lymphocytes of the neoplastic cell line MT-4 [8] infected with HIV-1, in the presence of DNA-Na-Fe.
Materials and methods
MT-4 cells were obtained from the collection of cell lines of the D.I. Ivanovsky Institute of Virology of the N.F. Gamaleya Research Center for Epidemiology and Microbiology of the Ministry of Health of Russia. Cells were cultured in RPMI 1640 medium containing 10% bovine fetal serum, 2 mM L-glutamine and 50 µg/mL gentamicin in an atmosphere of 5% CO2 at +37 °C. Cells were reseeded after 3–4 days, the density at receeding was 2.5–3.0 × 105 cl/mL. The HIV-1/899A strain obtained from the virus collection of the D.I. Ivanovsky Institute of Virology, N.F. Gamaleya Research Center for Epidemiology and Microbiology of the Ministry of Health of Russia was used for infection. The virus was passivated on cells in 50 ml culture vials for 5–7 days until the development of a pronounced cytopathic effect detectable under a light microscope. The culture supernatant was then withdrawn and the infection titer, expressed as log10 TCID50/mL (50% tissue cytopathic infectious dose), was determined. Sample aliquots were stored at −80 °C until use. Cells were infected with virus at a multiplicity of infection of about 100 TCID50/cell. To study the effect of DNA-Na-Fe on the expression of surface proteins, the preparation up to a final concentration of 500 mg/mL (developed by PharmPak LLC, manufactured by Immunoplex LLC, the commercial solution contains 15 mg of sodium deoxyribonucleate and 0.048 mg of iron oxide chloride in 5 ml of water for injection). At 48 and 72 h after culturing, cell viability was determined in the presence of trypan blue and aliquots were taken for p24 protein quantitation by enzyme-linked immunosorbent assay using a commercial test system (Genscreen ULTRA HIV Ag-AB, Bio-Rad, France). The level of inhibition of viral activity in percent was determined by the formula:
(ES – CV / CC − VC) × 100%,
where: ES – optical density readings of experimental samples with drug; VC – optical density readings of virus control (without drug); CC – cell control readings.
The readings of the well containing no cells were automatically subtracted when determining the optical density of the test samples. Experimental samples were tested in three parallel runs.
To analyze external phenotypic markers, cells were stained with monoclonal antibodies from Beckman Coulter (USA): CD4 (PE or PC5), CD28 (PC5), CD38 (PC5), CD62L(PE), HLA-DR (PE), IgG1 (PE), IgG1 (PC-5), IgG2a (PE). The cell suspension was pre-washed 3 times in 0.01M phosphate-salt buffer solution (pH 7.2) by centrifugation at 800 rpm for 6 min. Then suspended in the same solution at a concentration of 2 × 106 cells/mL. The stained cells were analyzed on an EPICS XL flow cytofluorimeter (Beckman Coulter, USA). The obtained histograms were processed using Kaluza program (Beckman Coulter, Software Version 1.2, USA).
Statistical analysis of data was performed using BioStat v.5 (AnalystSoft, USA) and Kaluza program. The level of significance (α) was equal to 0.05, the fluctuations of the coefficients of variation in all studied samples in the comparative analysis of cytometry results were within the normal range (> 10%).
Results
Most plasma membrane proteins of immune cells are multifunctional and their activity can be affected by many factors. MT-4 cells are neoplastic CD4+ T-lymphocytes transformed by human T-lymphotropic virus type 1 (Retroviridae: Orthoretrovirinae: Deltaretrovirus: Human T-lymphotropic virus type 1, HTLV-1). And this feature of them, as well as HIV-1, may be the cause of fluctuations in the expression of various regulatory proteins of the cell in general and membrane proteins in particular. It should be noted that when analyzing control cells in our studies, such indicators of membrane protein expression as the number of CD4+, CD25+, CD95+ and HLA-DR+ were unchanged. Practically about 100% of cells contained these markers [9]. Significant variations were observed in the amount of other markers. Virus-producing activity of cells in the process of long-term cultivation can also change. Therefore, in this work we used two variants of cells that were frozen at different periods of cultivation, simultaneously raised from nitrogen and labeled as L1 and L2. The properties of cells of the L1 line modified by two alleles of the ссr5 gene were described previously [10]. Cell infection and staining with monoclonal antibodies were performed under identical conditions in the same experiment. Cell viability 48 and 72 h after analysis of all experimental samples was 95% or more. In all experiments, the level of viral activity without the addition of the drug was almost the same 48 h after infection; after 72 h, the virus activity was on average approximately 1.2-fold higher in L1. As a rule, the maximum viral activity in the virus-cell model we used was observed on the 5th–6th day after infection. The study of the antiviral activity of DNA-Na-Fe showed that the level of inhibition of viral activity was 32 ± 1.7 and 49.6 ± 1.3% in the L1 cell line, and 13.8 ± 2 and 26.7 ± 1.4% in L2 cells 48 and 72 h after infection, respectively.
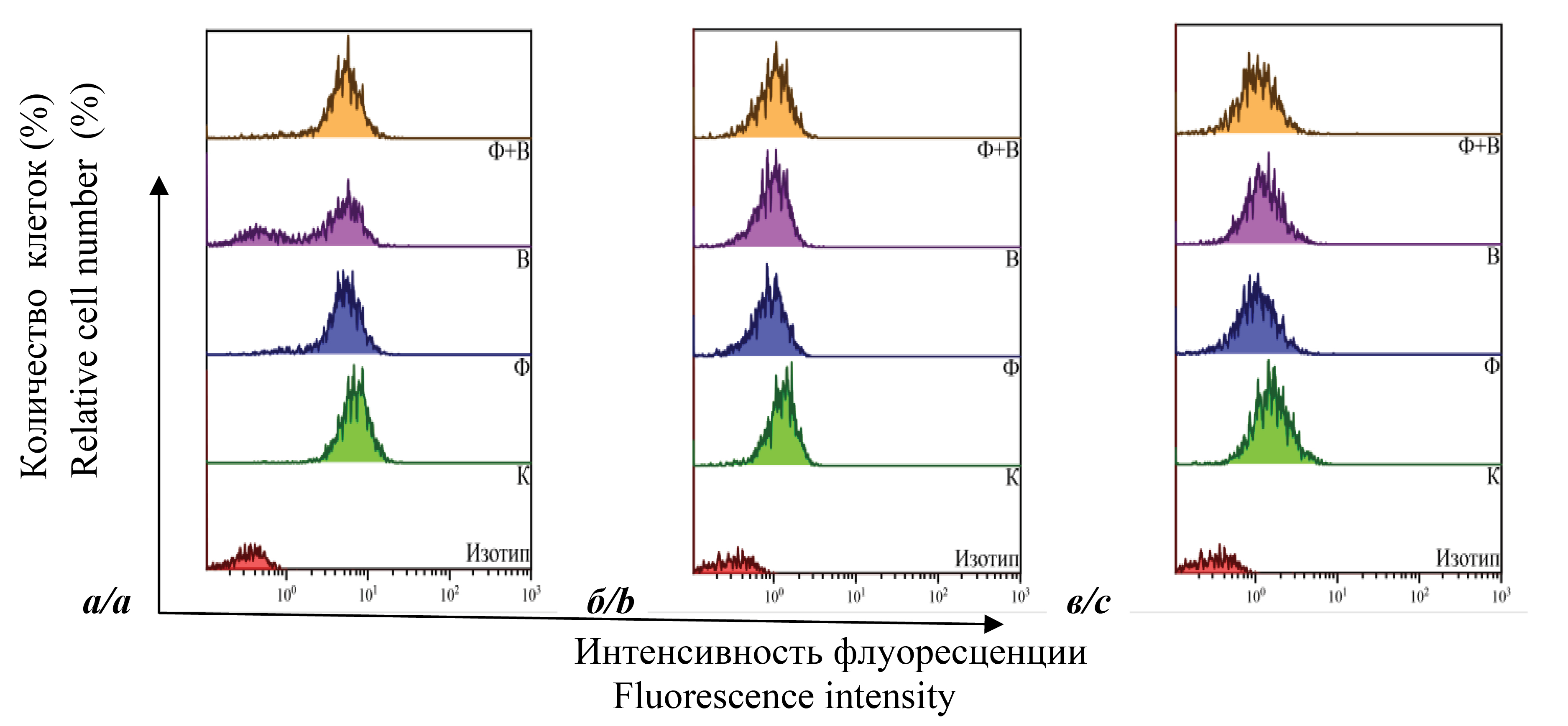
Figure 1 shows the results of determining the number of cells containing the following surface markers – CD4+, CD28+, CD38+, CD62L+ and HLA-DR+. Uninfected cells (K); uninfected cells cultured in the presence of DNA-Na-Fe (F); infected cells (B); and infected cells cultured in the presence of DNA-Na-Fe (F + B) were analyzed. The figure shows that 48 h after infection, the number of CD4+ cells in all samples of the two lines was almost the same, ranging from 94 to 99%. The presence of DNA-Na-Fe in samples F and F + B did not change the value of the index. No significant changes were also observed in fluorescence intensity (FI; indicator of marker expression density or mean fluorescence intensity). At 72 h after infection, the quantitative index was also essentially unchanged for all samples except B. This index decreases significantly for L1 (approximately 27%) and slightly (~ 5%) for L2. In the presence of DNA-Na-Fe, it increased and was almost the same for samples K and F. The results for samples L1 are presented as one-parameter histograms in Figure 2 (a). It can be seen that some of the cells in sample B lose this marker, and the addition of DNA-Na-Fe eliminates the effect. The histograms also show that the drug decreased the FI of uninfected cells, while the increase in the number of infected cells carrying CD4+ cells in sample F + B was accompanied by an increase in FI and corresponded to sample F. Similar dynamics was observed in the FI of CD4+ cells in samples L2.
Fig. 1. The results of determining the number of cells containing surface markers of MT-4 cells using the flow cytometry method. a – L1, b – L2. K – control cells; F – cells cultured with DNA-Na-Fe; B – infected cells; F + B – infected cells cultured with DNA-Na-Fe.
Рис. 1. Результаты определения количества клеток, содержащих поверхностные маркеры клеточной линии МТ-4, с использованием метода проточной цитометрии. а – Л1, б – Л2. К – контрольные клетки; Ф – клетки, культивируемые с ДНК-Na-Fe; В – зараженные клетки; Ф + В – зараженные клетки, культивируемые с ДНК-Na-Fe.
Fig. 2. Single-parameter histograms of the distribution of cells containing surface markers CD4, CD28, CD38. CD4+ – 72 hours after infection L1 (а); CD28+ – 48 hours after infection L1 (b); CD38+ – 48 hours after infection L1 (c). K – control cells; F – cells cultured with DNA-Na-Fe; B – infected cells; F + B – infected cells cultured with DNA-Na-Fe.
Рис. 2. Однопараметрические гистограммы распределения клеток, содержащих поверхностные маркеры CD4, CD28, CD38. CD4+ – через 72 ч после заражения Л1 (а); CD28+ – через 48 ч после заражения Л1 (б); CD38+ – через 48 ч после заражения Л1 (в). К – контрольные клетки; Ф – клетки, культивируемые с ДНК-Na-Fe; В – зараженные клетки; Ф + В – зараженные клетки, культивируемые с ДНК-Na-Fe.
As shown in Fig. 1, there are differences in the number of CD28+ cells 48 and 72 h after culturing. For the L1 cell line, its significant decrease relative to K was registered in all samples after 48 h. After 72 h this index decreased relative to control in sample F and the greatest decrease (~ 64%) was observed in sample B. For sample F + B it increased to the level of K. For L2 cells, after 48 h, slight fluctuations in the number of CD28+ cells relative to control were recorded; after 72 h, this index decreased by approximately 10.0 ± 2.6% in all experimental samples. Noticeable changes in FI were observed only in L1 after 48 h (Fig. 2 b). It can be seen that in all experimental samples the FI is lower than the control and almost identical for all experimental samples. Thus, we can conclude from the expression level of CD28 that DNA-Na-Fe reduces the number of cells containing this marker. Also, infection of cells leads to a decrease in the expression of this protein, but a clear effect was evident 72 h after infection. No significant regularities in the level of CD28 protein expression in infected cells in the presence of DNA-Na-Fe could be revealed. But this index was lower or comparable to the K samples.
A decrease in the amount of CD38+ protein for both cell lines was observed in the majority of experimental samples (Fig. 1). The largest was for L1 in infected cells after 72 h (~ 52%). Otherwise, the decrease in expression of this protein in infected cells was as follows: about 20% in L1 48 h after infection and about 6% after 72 h in L2, or no change (after 48 h in L2). In both cell lines, the addition of DNA-Na-Fe reduced the expression level of this protein between about 27–40% throughout the cell culture period. And this index did not change significantly when compared with samples in which cells were infected with the drug, the difference was ± 5%. Meanwhile, the expression of CD38 protein in infected cells upon DNA-Na-Fe addition in all samples was significantly lower than K (by approximately 22–37%). A stable decrease in FI in all samples was observed only in L1, and was more pronounced after 48 h (Fig. 2 c). In L2, this index was almost equal to K in F + B samples, and a slight decrease of approximately 7–9% was recorded in samples where the drug was present. As can be seen, this index was lower in all samples relative to sample K. Thus, it can be concluded that DNA-Na-Fe reduced the expression of CD38 protein in uninfected and infected cells.
The study of CD62L protein expression showed that in L1 in all samples there was an increase in cells carrying this marker compared to the control 48 and 72 h after infection (Fig. 1 a). In uninfected cells, DNA-Na-Fe increased this marker approximately 3-fold compared to K. A significant increase was observed for sample B after 48 h (~ 4-fold) and a non-significant increase (~ 1.2-fold) after 72 h. In the F + B sample, it was comparable to the F reading after 48 h, lower than the F reading and higher than the B reading after 72 h. An increase in FI was also observed in all L1 samples (Fig. 3 a, b). The exception was the sample B 72 h after infection (Fig. 3 b). The results obtained for L2 are difficult to interpret because the number of CD62L+ cells was negligible. It can only be noted that, in general, the kinetics was similar to L1.
Fig. 3. Single-parameter histograms of the distribution of cells containing surface markers CD62L and HLA-DR. CD62L+ – L1 48 hours after infection (a) and 72 hours after infection (b); HLA-DR+ – 48 hours after infection L1 (c) and L2 (d), L2 72 hours after infection (e). K – control cells; F – cells cultured with DNA-Na-Fe; B – infected cells; F + B – infected cells cultured with DNA-Na-Fe.
Рис. 3. Однопараметрические гистограммы распределения клеток, содержащих поверхностные маркеры CD62L и HLA-DR. CD62L+ – Л1 через 48 ч после заражения (а) и 72 ч после заражения (б); HLA-DR+ – через 48 ч после заражения Л1 (в) и Л2 (г), Л2 через 72 ч после заражения (д). К – контрольные клетки; Ф – клетки, культивируемые с ДНК-Na-Fe; В – зараженные клетки; Ф + В – зараженные клетки, культивируемые с ДНК-Na-Fe.
The number of HLA-DR+ cells for both lines after 48 and 72 h was about 99 ± 5% for all samples. With regard to FI, the following significant changes should be noted. For L1, this index increased after 48 h in all samples relative to control (Fig. 3 c), but in samples with DNA-Na-Fe (F and F + B) to a greater extent than in sample B. After 72 h, FI was not significantly increased in sample F and F + B and decreased in sample B. Analysis of L2 samples showed the following values relative to control. After 48 h, DNA-Na-Fe decreased FI. The presence of virus significantly increased this index. In the F + B sample, this index decreased but was higher than for the K and F samples (Fig. 3 d). After 72 h, FI was lower than in the control in all experimental samples. The decrease was most pronounced in sample B, and to a lesser extent in sample F + B. DNA-Na-Fe decreased this index in sample F insignificantly (Fig. 3 e).
Discussion
The peculiarities of HIV-1 replication are currently very well studied [11]. The membrane protein CD4 of T-lymphocytes is the main receptor used by the virus in the early stages of infection. It is known that during virus replication, the number of CD4+ in infected cells decreases. This is a mechanism used by the virus to facilitate the budding of mature viral particles involving the viral proteins nef and vpu. When analyzing the expression of this protein in two cell lines, a pronounced effect of decreasing the number of CD4+ was observed only in cells of line L1 72 h after infection, probably at the beginning of the development of the active phase of budding of viral progeny (Fig. 1 a, Fig. 2 a). But a decrease in FI was recorded in both lines. This indicates that in L2 the virus-specific effect of CD4 level reduction was also present, but was weaker. This is most likely due to the fact that viral activity in L1 has been shown to be higher than in L2. It is possible that this effect would have been more pronounced at later stages of infection. Infection of cells in the presence of DNA-Na-Fe resulted in a restoration of CD4+ cell counts to control levels and an increase in FI (Fig. 1 a, Fig. 2 a), which contributed to a decrease in virus yield. Virus-producing activity of cells in the presence of DNA-Na-Fe was reduced in both cell lines. The antiviral effect was higher in the system with more active virus replication. This suggests that its use may be more effective at the stages of HIV infection with a high viral load – at the stage of acute infection and the late stage of AIDS. Thus, it can be assumed that the enhancement of CD4 expression by infected cells in the presence of DNA-Na-Fe is associated with the ability of the drug to influence the mechanisms that alter the metabolism of this protein and/or reduce the activity of viral proteins nef and vpu. It should be noted that in uninfected cells the drug reduced FI relative to the control (Fig. 2 a). This fact also suggests an effect of DNA-Na-Fe on CD4 protein metabolism, which thus may lead to a decrease in the sensitivity of cells to infection. It cannot be excluded that DNA-Na-Fe has the ability to activate antiviral cellular factors.
CD4 T cells play an important role in the development and coordination of adaptive immunity. Their activation is necessary for the organization of an effective immune response. An essential component of T-cell activation is the costimulatory membrane protein CD28, which excludes the development of an aberrant immune response. CD28 regulates a wide range of cellular processes, from proliferation and survival to promoting differentiation of specialized subpopulations of T cells. Since its first discovery more than 20 years ago, CD28 has remained the subject of intense research due to its importance for normal T cell function and its potential as a therapeutic target [12, 13]. It has been shown that, as in the case of the viral CD4 receptor, its amount on the cell membrane is reduced upon viral infection with the involvement of the same viral proteins, nef and vpu [14]. Our studies showed that the virus significantly reduced the expression of this protein in cell line L1 cells 48 and 72 h after infection, and the addition of DNA-Na-Fe 72 h after infection eliminated this effect (Fig. 1 b). In L2, broadly similar dynamics were observed. However, the effects observed for L2 were more weakly expressed. This is apparently also due to the reduced viral activity in the cells. In general, we can conclude that the antiviral activity of DNA-Na-Fe is associated with changes in CD28 metabolism, as in the case of CD4. 72 h after infection, its amount in L1 in the F + B sample was restored to the control level. This feature of it in the in vivo system may contribute to the increased immunity of infected T cells. DNA-Na-Fe also reduced the number of CD28+ cells in control samples in both lines, indicating its immunomodulatory properties expressed as a decrease in the activation of the immune system. Which, in turn, may reduce susceptibility to various infections. As for the possible effect of DNA-Na-Fe on the reduction of costimulatory ability of normal cells, this requires additional research. An increased level of CD28 protein is an unfavorable prognostic factor in various lymphomas [15]. In the normal body, there is a balance between stimulatory and inhibitory signals that determine the ultimate nature of T-cell responses and can be driven by multiple factors, including complex receptor-ligand interactions between CD28 family members depending on cell type. Despite the central role of CD28, its family members and ligands for immune function, many aspects of CD28 biology remain unclear. The introduction of a basic understanding of CD28 function in immunomodulatory therapies has been controversial, with successes and failures. It cannot be ruled out that DNA-Na-Fe may assist in the regulation of costimulatory signaling and serve as an additional regulator of maintaining an appropriate balance. The different effect of DNA-Na-Fe on CD28 protein expression in L1 cells and the non-significant effect in L2 cells indicates a complex mechanism of regulation of genome expression in MT-4 cells in normal and HIV infection. The timely turning on and off of specific genes in certain cells causes their physiological changes. In general, we can conclude that 72 h after infection the number of cells expressing CD28+ protein significantly decreases, and the presence of DNA-Na-Fe in infected cells eliminates the viral effect and restores this index to normal. Thus, it should be noted that the drug decreased the level of activation of uninfected cells, but increased the amount of CD28+ protein in infected cells. At the same time, FI decreased compared to the control cells and was restored after 72 h of culturing infected cells in the presence of the drug. Since CD28 fulfills an important costimulatory role in the transmission of immune signals by the T-cell receptor, it can be concluded that DNA-Na-Fe can restore the immune potential of infected cells, which is reduced in HIV infection.
One of the important indicators of immune system activation in HIV infection is the membrane protein CD38. It is a multifunctional transmembrane protein whose expression level on the surface of immune cells depends on their activation. It plays a key role in signaling regulation of intracellular calcium level and modulation of survival and metabolic processes [16, 17]. The data accumulated in almost four decades since its discovery indicate that CD38 plays an important role in the functioning of various immune cells both in normal and in various pathologies. Therefore, it serves as a prognostic marker and a promising therapeutic target. CD38 has been shown to interact at the cell membrane with HIV-1 CD4 and gp120 proteins. This, in turn, may influence virus-cell interactions. Overall, there is considerable disagreement when analyzing data on the role of CD38 in the immune response in HIV infection. And the use of its antagonists for therapeutic purposes has not given real encouraging results. Thus, despite the wealth of data on the diverse functions of CD38 in the immune response, new and more sophisticated approaches are needed to determine the consequences of targeting specific CD38-mediated activity during the host response to infection.
Our data obtained using two variants of the cell line showed that in samples of both lines in the presence of DNA-Na-Fe in uninfected and infected cells there was a significant decrease in CD38 protein expression compared to control (Fig. 1). In infected L1 cells, the decrease was lowest after 48 h (~ 20%) and highest after 72 h (~ 50%), while in L2 it was unchanged or about 20%, respectively. Based on these data, it can be assumed that after 72 h the activation of the process of budding of viral progeny begins. And at this stage, the mechanisms of its expression reduction by viral factors are manifested, as in the case of other cell membrane proteins capable of interacting with gp120 and preventing budding. In the L2 cell line this effect is less pronounced, also apparently due to reduced viral activity. As shown by the results on the reduction of virus replication in F + B samples, DNA-Na-Fe, by reducing cell activation, probably contributed to the reduction of virus replication as well.
The outer cell membrane protein CD62L is a cell adhesion molecule that ensures the process of leukocyte circulation. The protein plays an essential role in the transport of activated lymphocytes to lymphoid organs and the initiation and maintenance of immune response against pathogens during the development of infection. The CD62L protein is known to facilitate virus entry into cells by assisting the adhesion of the virus gp120 protein with the CD4 receptor and co-receptor. However, during budding, the amount of CD62L must be reduced for successful release of particles into the environment, as it retains the viral particles on the cell membrane. Therefore, when lymphocytes are infected with HIV-1, its expression is reduced with the involvement of the viral proteins nef and vpu. These proteins bind to CD62L in the cell and prevent its transport to the membrane. The virus uses this protein in early stages of infection to attach to cells, and in later stages induces outer domain segregation or endocytosis to facilitate budding of viral progeny [18, 19]. Decreased expression of this protein on the cell membrane leads to decreased circulation and activation of infected cells and contributes to the intensification of the infectious process and impaired development of the adaptive immune response.
The study of CD62L protein expression in L1 and L2 lines showed that, in general, the dynamics of readings in the experimental samples was similar, but in L2 it was significantly lower than in L1. This, apparently, as well as for the markers described above, is due to the peculiarities of cells and their virus-producing activity. Therefore, it is necessary to dwell on the results obtained for L1. In all samples, an increase in CD62L expression was observed compared to the control both in number and in FI (Fig. 1 a; Fig. 3 a, b). It should be noted that in infected cells, these values were almost equal to the control after 72 h (Fig. 1 a) or lower (Fig. 3 b). The latter result indicates that the virus is able to reduce the expression of CD62L.
Based on these data, we can conclude that the effect of DNA-Na-Fe may be twofold. On the one hand, the drug can enhance the infection of cells by the virus. On the other hand, the increased expression of CD62L protein in the presence of the drug indicates that DNA-Na-Fe is able to reduce HIV-1 replication in the in vitro system. Therefore, the observed effect of reducing HIV replication in the presence of DNA-Na-Fe can be explained by an increase in CD62L protein expression. The first negative effect may be offset in vivo by the currently known cellular mechanisms controlling HIV-1 infection and replication [1]. The difference in the effect of the drug on CD62L protein expression in MT-4-cells observed in different cell lines indicates that CD62L does not have a high specific activity and its effect probably depends on the peculiarities of the aggregate molecular and biological processes occurring in the cells.
The immune cell marker HLA-DR (class II histocompatibility antigen) is a major marker of lymphocyte and monocyte activation that was found in CD4 cells from patients with T-cell leukemia associated with HTLV-1 infection [20]. It regulates calcium content in cell cytoplasm, cell membrane permeability, and activates phosphorylation of intracellular proteins. HLA-DR protein is closely related to the immune response, regulation of cell responses to external stimuli and is of great importance in the development of autoimmune diseases, tumor immunity, and organ transplantation. In HIV infection, a special role is given to the study of HLA-DR+ T-lymphocytes, as they have increased proliferative activity [21]. Of particular importance is the pool of infected memory cells containing active provirus, which persists with active ART and is a source of relapses [22].
As our studies have shown, in all studied samples of two cell lines practically more than 90% of cells were HLA-DR+ (Fig. 1 a, b). And this indicator did not change relative to the control. And, if no difference was observed in quantitative terms, the FI index changed by the time of cultivation (Fig. 3 c–e). However, the results obtained are contradictory and do not allow us to draw any significant conclusions. It can only be noted that both DNA-Na-Fe and virus can alter the protein expression level both individually and in complex. We have previously shown that prolonged cultivation of MT-4 cells with DNA-Na-Fe resulted in a decrease in the number of HLA-DR+ cells [7].
In general, the observed differences in the effect of DNA-Na-Fe on cells and virus replication and on the expression of all studied surface proteins in the presence of the drug in different cell lines indicate that they depend on the peculiarities of the aggregate molecular-biological processes occurring in cells. The differences in the effect of DNA-Na-Fe on HIV replication observed in systems with different replicative activity indicate its more active participation in cell metabolism and alteration of virus replication processes at the stages of penetration and budding of progeny virions in more active systems and/or only at certain ratios of all participants in the metabolic process.
Conclusion
The studies showed that DNA-Na-Fe has antiviral and immunomodulatory activity. Based on the results obtained, it can be concluded that the antiviral activity of the drug in the infection of MT-4 cells with HIV-1 is associated with its immunomodulatory activity, which changes the expression of membrane proteins CD4, CD28, CD38 and CD62L. The mechanism of HIV-1 replication reduction in the presence of DNA-Na-Fe can be explained by its participation in regulating the level of CD4 receptor in infected cells, as well as proteins that are markers of cell activation.
Funding. This study was not supported by any external sources of funding.
Conflict of interest. Sodium deoxyribonucleate with iron complex was provided by LLC «PharmPak», Russia. The authors' collective includes an affiliated employee of the developer company, PharmMPark LLC, who is not the CEO. PharmMPark LLC did not act as a sponsor of the study.
About the authors
Dmitry N. Nosik
FSBI «National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya» of the Ministry of Health of Russia
Author for correspondence.
Email: dnnosik@yandex.ru
ORCID iD: 0000-0001-5757-5671
Professor, Doctor of Medical Sciences, Head of the Laboratory of Antiviral and Disinfection Agents, The D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowLyudmila B. Kalnina
FSBI «National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: klb3@yandex.ru
ORCID iD: 0000-0002-2702-8578
Candidate of Biological Sciences, Leading researcher of the Laboratory of Antiviral and Disinfection Agents, The D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowLyudmila M. Selimova
FSBI «National Research Centre for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya» of the Ministry of Health of Russia
Email: lselim@mail.ru
ORCID iD: 0000-0003-3709-770X
Doctor of Biological Sciences, Leader Researcher at the Laboratory of Antivirals and Disinfectants, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowEllie N. Kaplina
LLC «PharmPak»
Email: info@derinat.ru
ORCID iD: 0000-0001-8540-5856
Candidate of Technical Sciences, Professor RAE, Consultant
Russian Federation, 105318, MoscowReferences
- Masenga S.K., Mweene B.C., Luwaya E., Muchaili L., Chona M., Kirabo A. HIV-host cell interactions. Cells. 2023; 12(10): 1351. https://doi.org/10.3390/cells12101351
- Hokello J., Tyagi P., Dimri S., Sharma A.L., Tyagi M. Comparison of the biological basis for non-HIV transmission to HIV-exposed seronegative individuals, disease non-progression in HIV long-term non-progressors and elite controllers. Viruses. 2023; 15(6): 1362. https://doi.org/10.3390/v15061362
- Ventura J.D. Human Immunodeficiency Virus 1 (HIV-1): Viral latency, the reservoir, and the cure. Yale J. Biol. Med. 2020; 93(4): 549–60.
- Lv T., Cao W., Li T. HIV-related immune activation and inflammation: current understanding and strategies. J. Immunol. Res. 2021; 2021: 7316456. https://doi.org/10.1155/2021/7316456
- Yang X., Su B., Zhang X., Liu Y., Wu H., Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020; 107(4): 597–612. https://doi.org/10.1002/JLB.4MR1019-189R
- Besednova N.N., Makarenkova I.D., Fedyanina L.N., Avdeeva Zh.I., Kryzshanovsky S.P., Kuznetsova T.A., et al. Prokaryotic and eukaryotic DNA in prevention and treatment of infectious diseases. Antibiotiki i khimioterapiya. 2018; 63(5-6): 52–67. https://elibrary.ru/xvvinv (in Russian)
- Nosik D.N., Kalnina L.B., Lobach O.A., Chataeva M.S., Berezhnaya E.V., Bochkova M.S., et al. Antiviral and virucidal activity of sodium deoxyribonucleate and its complex with iron against viruses of different kingdoms and families. Voprosy virusologii. 2022; 67(6): 506–15. https://doi.org/10.36233/0507-4088-148 https://elibrary.ru/rtrade (in Russian)
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., et al. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981; 294(5843): 770–1. https://doi.org/10.1038/294770a0
- Selimova L.M., Kalnina L.B., Nosik D.N. The superficial markers of neoplastic cell line mt-4 and perspectives of its application as a model for studying activity of immune modulating preparations. Klinicheskaya laboratornaya diagnostika. 2016; 61(12): 822–5. https://doi.org/10.18821/0869-2084-2016-61-12-822-825 https://elibrary.ru/xscfqz (in Russian)
- Nosik D.N., Kalnina L.B., Selimova L.M., Pronin A.V. An increase in the infectivity of the human immunodeficiency virus with modification of the ccr5 gene receptor of sensitive cells. Doklady Rossiiskoi Akademii nauk. Nauki o zhizni. 2023; 511(1): 344–8. https://doi.org/10.31857/S2686738923700257 https://elibrary.ru/jiltbd (in Russian)
- Levesque K., Finzi A., Binette J., Cohen E.A. Role of CD4 receptor down-regulation during HIV-1 infection. Curr. HIV Res. 2004; 2(1): 51–9. https://doi.org/10.2174/1570162043485086
- Boomer J.S., Green J.M. An enigmatic tail of CD28 signaling. Cold Spring Harb. Perspect. Biol. 2010; 2(8): a002436. https://doi.org/10.1101/cshperspect.a002436
- Nandi D., Pathak S., Verma T., Singh M., Chattopadhyay A., Thakur S., et al. T cell costimulation, checkpoint inhibitors and anti-tumor therapy. J. Biosci. 2020; 45: 50.
- Leonard J.A., Filzen T., Carter C.C., Schaefer M., Collins K.L. HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J. Virol. 2011; 85(14): 6867–81. https://doi.org/10.1128/JVI.00229-11
- Rohr J., Guo S., Huo J., Bouska A., Lachel C., Li Y., et al. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia. 2016; 30(5): 1062–70. https://doi.org/10.1038/leu.2015.357
- Glaría E., Valledor A.F. Roles of CD38 in the Immune Response to Infection. Cells. 2020; 9(1): 228. https://doi.org/10.3390/cells9010228
- Savarino A., Bottarel F., Malavasi F., Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions? AIDS. 2000; 14(9): 1079–89. https://doi.org/10.1097/00002030-200006160-00004
- Vassena L., Giuliani E., Koppensteiner H., Bolduan S., Schindler M., Doria M. HIV-1 Nef and Vpu interfere with L-selectin (CD62L) cell surface expression to inhibit adhesion and signaling in infected CD4+ T lymphocytes. J. Virol. 2015; 89(10): 5687–700. https://doi.org/10.1128/JVI.00611-15
- Segura J., He B., Ireland J., Zou Z., Shen T., Roth G., et al. The role of L-selectin in HIV infection. Front. Microbiol. 2021; 12: 725741. https://doi.org/10.3389/fmicb.2021.725741
- Freedman A.S., Nadler L.M. Immunologic markers in non-Hodgkin’s lymphoma. Hematol. Oncol. Clin. North Am. 1991; 5(5): 871–89.
- Mahalingam M., Pozniak A., McManus T.J., Vergani D., Peakman M. Cell cycling in HIV infection: analysis of in vivo activated lymphocytes. Clin. Exp. Immunol. 1995; 102(3): 481–6. https://doi.org/10.1111/j.1365-2249.1995.tb03841.x
- Horsburgh B.A., Lee E., Hiener B., Eden J.S., Schlub T.E., von Stockenstrom S., et al. High levels of genetically intact HIV in HLA-DR+ memory T cells indicates their value for reservoir studies. AIDS. 2020; 34(5): 659–68. https://doi.org/10.1097/qad.0000000000002465
Supplementary files