Evaluation of anti-HIV-1 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus type 1) activity of 6HP and 3TC in vitro using MT-4 cell line variants with different replicative activity
- Authors: Kalnina L.B.1, Selimova L.M.1, Nosik D.N.1
-
Affiliations:
- The D.I. Ivanovsky Research Institute of Virology the N.F. Gamaleya NRCEM of the Ministry of Health of the Russian Federation
- Issue: Vol 69, No 5 (2024)
- Pages: 441-448
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16669
- DOI: https://doi.org/10.36233/0507-4088-247
- EDN: https://elibrary.ru/ptyvcq
- ID: 16669
Cite item
Abstract
Introduction. Chemotherapy of HIV infection remains the only means of treating the disease. The process of development new and improving previously developed drugs is therefore considered a priority. One of the preclinical stage of drug efficacy testing is research in the virus-cell model system in vitro.
The aim. To evaluate the antiviral efficacy of nucleoside reverse transcriptase inhibitors (NRTIs) 6HP and 3TC during HIV-1 replication in the neoplastic MT-4 cell line.
Materials and methods. Two variants of the CD4+ T-lymphocyte MT-4 cell line (MT-4/1 and MT-4/2) transformed by Human T-lymphotropic virus type 1 (Retroviridae: Orthoretrovirinae: Deltaretrovirus: HTLV-1), with different levels of HIV-1 replication were used. Drugs ammonium-3’-azido-3’-deoxythymidine-5’-carbomoylphosphonat (6HP) and 2’,3’-dideoxy-3’-thiacytidine (3TC) were used to suppress the virus.
Results and discussion. The replication activity of HIV-1 was observed to be higher in the MT-4/2 line than in the MT-4/1 line for different strains of the virus. The use of each of the substances separately showed a more significant inhibition of viral activity in MT-4/1 than in MT-4/2 cells. When used together, the inhibition level was almost the same in all cases and ranged from 87‒96% for the MT-4/1 line and 83‒89% for the MT-4/2 line. High efficacy was observed when using lower concentrations of drugs compared to individual use.
Conclusion. The combined use of NRTIs 6НР and 3TС is promising for the treatment of HIV-infected patients at different stages of infection and with different levels of viral load.
Keywords
Full Text
Introduction
Antiretroviral therapy (ART) has been successfully used to treat HIV infection and can effectively suppress viral replication. Millions of lives have been saved as a result of the worldwide implementation of a systematic approach to treating the infection. Since 2001, 16.5 million AIDS-related deaths have been averted. According to UNAIDS1, investment in maximizing the engagement of HIV-infected people in ART has resulted in 27.5 million (73%) of the 37.7 million people living with HIV-1 receiving treatment by the end of 2022. Treatment coverage in 2023 in Russia amounted to 86.5% of those on dispensary care and 58.8% of those living with HIV-1 infection.
However, for a number of reasons, positive effect is not achieved in all cases [1]. Given the complex pathogenetic features of the infection and the costs associated with lifelong treatment, the development of effective therapeutic approaches remains a priority at present, as before. Therapeutic regimens that include the use of three drugs that inhibit the virus at different replication stages are considered to be more effective. A review of the most significant studies that included two drugs for initial treatment of HIV-1 or to maintain high levels of viral replication suppression at the asymptomatic stage demonstrated that, after more than two decades of using three drugs, there may be a shift in priorities in the near future. For certain populations where drug toxicity needs to be minimized, where drug intolerance exists, or where a simpler drug regimen is needed, two-drug therapy may be considered a safe and effective alternative [2].
The first group of drugs capable of inhibiting viral replication at the reverse transcription stage [3] were nucleoside reverse transcriptase inhibitors (NRTIs). Among them, the first compound approved for the treatment of HIV-1 [4], the thymidine analog 3’-azido-3’-deoxythymidine (AZT, international name zidovudine), still frequently serves as a component of many NRTI regimens and forms the basis of available antiretroviral therapies. However, the marked toxicity of AZT and its ability to generate strains carrying multiple mutations leading to resistant variants of the virus with reduced sensitivity to it [5] have stimulated the search for new dosage forms.
Among the successful studies on the search for such drugs were the works of Russian scientists on the creation of phosphonate derivatives of reverse transcriptase inhibitors based on AZT. First of all, this is 3’-azido-3’-deoxythymidine-5’-monophosphate sodium (phosphazide), an original Russian anti-HIV drug, which passed the full scope of preclinical and clinical trials and was registered in the Russian Federation in 1999. The drug showed high efficacy, reduced toxicity, prolonged action and delayed formation of resistant HIV-1 strains [6]. Further studies resulted in a new drug ammonium-3’-azido-3’-deoxythymidine-5’-carbomoylphosphonate (6NP). This compound has pronounced antiviral properties and favorable pharmacokinetic parameters. In the body, 6NP is efficiently converted to AZT. Slow release after oral administration and penetration into body cells, as well as reduced toxicity make this prodrug promising as a prolonged-release form of AZT with antiviral action [7].
The next NRTI related to cytidine analog was 2´,3´-dideoxy-3´-thiacytidine (3TC, international name lamivudine) [8]. This drug has been successfully used in the practice of HIV treatment for about 28 years. As new drugs are being certified, treatment regimens that include 3TC continue to be widely used in initial therapy due to its efficacy and relative safety. The drug is widely used to treat patients of all ages. Due to its minor drug interactions and low cost, 3TC continues to be used in new regimens in combination with next-generation antiretrovirals. It is also prescribed to patients with hepatitis B virus coinfection. Among all drugs first approved more than 20 years ago for the treatment of HIV infection, only 3TC is still recommended in the most recent internetional guidelines. However, as a component of various antiretroviral regimens, 3TC continues to be actively studied. The drug has a unique resistance profile, capable of slowing its development to AZT and possibly compensating for reduced sensitivity to AZT through its contribution to antiviral action. Furthermore, the phenomenon of enhanced antiviral effect is known for combination drugs with minimal doses of their ingredients, which by themselves are not able to cause a noticeable antiviral effect [9]. Combivir, a drug combining both substances, was created on the basis of AZT and 3TS and has been used since 1997. In 2018, the Russian drug “Fosfaladin” combining fosfazide and lamivudine was registered. The combination of drugs in a single tablet produces a stronger and more sustained effect than when they are used separately. It also helps reduce polypragmasy and facilitates adherence to medication adherence [10].
In connection with the development of a new drug named 6NR, it was important to conduct a more in-depth study on the effect of the combination of 6NR and 3TS on HIV-1 replication in an in vitro model system. Two varieties of neoplastic CD4+ T-cell line MT-4 with different replicative activity were used in this work. The phenotypic features of the lines were described earlier [11].
The aim of the study was to investigate the antiviral efficacy of NRTIs 6NR and 3TS on HIV-1 replication in the neoplastic MT-4 cell line.
Materials and methods
Human MT-4/1 and MT-4/2 cells were obtained from the collection of cell lines of the D.I. Ivanovsky Institute of Virology, N.F. Gamaleya NICEM, Federal State Institution of Medical Sciences of Russia Ministry of Health of Russia. For their cultivation we used RPMI 1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine and 50 μg/mL gentamicin in an atmosphere of 5% CO2 at 37 °C. Cells were crossed after 3‒4 days, and the density at crossing was 2.5‒3.0 × 105 cl/mL. HIV-1 strains 899A, IIIB, NOV and MS-1979 were used to infect the cells. The first three strains belong to subtype B, while the last strain belongs to subtype G. The virus strains were obtained from the virus collection of the D.I. Ivanovsky Institute of Virology, N.F. Gamaleya NICEM, Federal State Institution of Medical Sciences of Russia Ministry of Health of Russia. The viruses were passaged on cells in 50 ml culture vials for 5‒7 days until the development of a pronounced cytopathic effect detectable under a light microscope. The culture fluid was then sampled and the infection titer was determined, expressed as log10 TCID50/mL (50% tissue cytopathic infectious dose). Sample aliquots were stored at −80 °C until cells were infected. Cells were infected with viruses at a multiplicity of infection of approximately 100 TCID50/cell. Aliquots of cell suspension were taken daily and cell viability was determined in the presence of trypan blue. On the 5th-6th day after infection, culture fluid was collected and TCID50/mL was determined by the end-point dilution method using the MT-4/1 line; cell viability was measured by the MTT method [12].
In the antiviral activity study, cells were cultured in 96-well plates; preparations 6HP and 3TS (provided by AZT Pharma K.B., Russia) were added simultaneously with the virus. After 3 days, aliquots were taken to determine the amount of p24 protein by enzyme-linked immunosorbent assay using a commercial test system (Genscreen ULTRA HIV Ag-AB, Bio-Rad Company, France). The level of inhibition of viral activity in percent was determined by the formula:
(ES − OD / CC − OD) × 100%,
where ES – optical density readings of experimental samples with preparation; OD – optical density readings of virus control (without preparation); CC – cell control readings. The readings of the well containing no cells were automatically subtracted when determining the optical density of the test samples. Experimental samples for evaluation of viral activity and antiviral activity of drugs were tested in three parallel repeats each.
Statistical analysis of data was performed using the BioStat, v.5 program (AnalystSoft, USA).
Results
When two variants of the MT-4/1 and MT-4/2 cell lines were infected with HIV-1 strains IIIB, 899A, NOV and MS1974, it was observed under a light microscope that the infectious process in the MT-4/2 line developed significantly more actively. A pronounced cytopathic effect was observed for all listed virus strains, exceeding the similar effect in the MT-4/1 line. The results of infectious titer determination showed that for strain 899A it increased by about 4 orders of magnitude, IIIB – by about 3 orders of magnitude, NOV and MC1974 – by about 1 order of magnitude. Subsequently, strain 899A was chosen for cell infection as the most highly productive and most frequently used in our work. The results of cell viability during 899A virus replication at different time points after infection are presented in Fig. 1. It can be seen that the number of live cells in the MT-4/2 line already on the 2nd day after infection was significantly lower than in MT-4/1. On the 5th day the cytopathic activity of the virus in MT-4/2 line was about 20% higher.
Fig. 1. Cytopathic activity of the HIV-1/899A virus during replication in MT-4/1 and MT-4/2 cell lines.
Рис. 1. Цитопатическая активность вируса ВИЧ-1/899А при репликации в линии клеток МТ-4/1 и МТ-4/2.
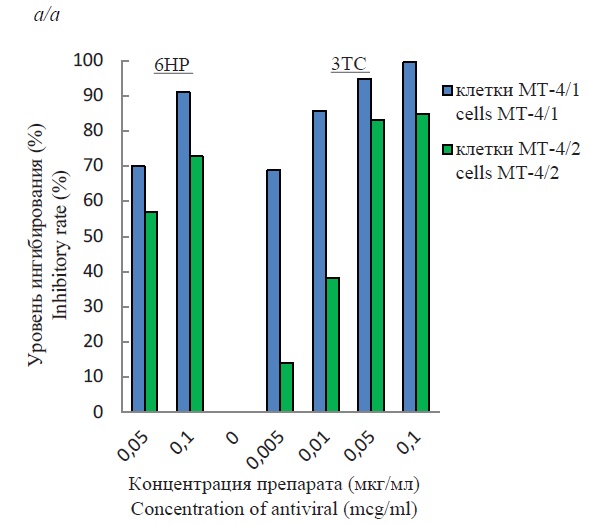
The presence of cell lines with different productive activity allowed us to study the efficacy of two antiviral drugs 6NR and 3TS that inhibit virus replication at the reverse transcription stage. The results of inhibition of HIV-1 replication by one drug and in combined use are presented in Fig. 2. In all cases, a dose-dependent antiviral effect was observed. As can be seen, the concentrations of each substance used individually resulted in a more significant inhibition of viral activity in MT-4/1 cells (Fig. 2 a). When the lowest concentrations of each drug were used, the percentage of inhibition of viral activity in MT/4-2 cells was significantly lower. Increasing the concentration of the drugs resulted in the inhibitory rate of MT-4/2 cells nearly reaching that of MT-4/1 cells, but remaining 10‒15% lower.
Fig. 2. Suppression of HIV-1/899A replication in MT-4/1 and MT-4/2 cell lines with compounds 6HP and 3TC.
Individual (a) and combined (b) action of antivirals.
Рис. 2. Подавление репликации ВИЧ-1/899А в линии клеток МТ-4/1 и МТ-4/2 препаратами 6НР и 3ТС.
Индивидуальное (а) и комбинированное (б) противовирусное действие.
When the substances were used together, the level of inhibition was high in all cases and almost similar, and ranged between 87‒96% for the MT-4/1 line and 83‒89% for the MT-4/2 line (Fig. 2 b). In statistical analysis of the results obtained, the significance level (p) was equal to 0.05.
Discussion
The presence of two varieties of the MT-4 lineage, in which virus productivity of the same HIV-1 strains was different, set us the task of identifying possible causes. Several approaches were used. First of all, we hypothesized that MT-4/2 cells may secrete soluble factors that can influence virus replication. In this regard, the effect of soluble factors of these cells on virus replication in the MT-4/1 line was studied [11]. The results of the performed experiments showed the absence of such.
MT-4 cells are represented by neoplastic CD4+ T-lymphocytes transformed by human T-lymphotropic delta retrovirus type 1 (Retroviridae: Orthoretrovirinae: Deltaretrovirus: Human T-lymphotropic virus type 1; HTLV-1). The infection is related to malignant neoplasms of the lymphoid and hematopoietic systems [13]. The cell line was derived from patients with adult T-cell leukemia/lymphoma. The cells are highly activated and serve as a convenient model for virologic and molecular biological studies. HIV-1 replication is known to occur in activated cells and therefore transformed cells serve as a convenient experimental model to study the replication features of this virus. Previously, the expression of activation markers by MT-4 cell line was studied [14]. However, the activation level of the two line variants used in the present study could be different. The main markers for assessing the activation potential of cells are the expression levels of external proteins CD28, CD38 and HLA-DR. Previous studies have shown that during culturing, the expression of HLA-DR in the lines was similar and amounted to 84‒99%. They differed in the expression level of CD28 and CD38. The most significant difference was observed for CD38 protein. After 72 h of cultivation, the number of cells expressing this protein was about 15 times higher in the high-yielding line [11]. CD38 is a transmembrane glycoprotein with receptor and enzyme functions [15]. Its biological role is believed to be completely unexplored. It was first detected as a receptor in activated cells and in the highest amount in tumor cells. It has now been shown that CD38 protein plays an essential role in cell metabolism and is a part of many biochemical processes at the cellular level. CD38 serves as a receptor in the regulation of cytokine release mechanisms, participates in signal transduction as part of receptor complexes, regulates cell adhesion in intermolecular interactions between cells and extracellular space and cell dissemination in the body. As a bifunctional enzyme, it controls extracellular nucleotide homeostasis and intracellular calcium fluxes. These functions are important in regulating the developmental features of pathologic processes in infection, oncogenesis, and aging. Possessing glycohydrolase activity, CD38 participates in energy storage in redox reactions using nicotinamideadenine dinucleotide coenzymes (NAD+ – oxidized form, NADH – reduced form). By combining the activities of adenosine diphosphate ribosyl (ADPR) cyclase and cyclic ADP-ribose (cADPR) hydrolase, the protein catalyzes the synthesis of cADPR, ADPR and nicotinic acid adenine dinucleotide phosphate (ADP-NA). These signaling molecules are involved in the mobilization of calcium ions ([Ca2+]) from intracellular depots of calcium channels of intracellular membrane structures (endoplasmic reticulum). Intracellular calcium is essential for many physiological and pathological processes occurring in blood cells, including degranulation, regulation of cytoskeleton protein interactions, activation of cellular kinases and phosphatases, transcriptional control, and modulation of surface receptors. Calcium has previously been shown to be essential in the early stages of HIV-1 virus entry into cells [16]. It cannot be excluded that calcium may play a role in all stages of virus-cell interaction, since the involvement of all intracellular membranes is inevitable during virus replication. Thus, it is possible that the increased expression of CD38 protein and its ability to regulate the level of intracellular calcium detected in the present study can explain the increased replicative activity of the virus in MT-4/2 cells.
There are other possible reasons for the increased activity of HIV-1. It has been shown that during HIV-1 replication, significant rearrangements occur in cells, accompanied by changes in membrane structure [17], a decrease in the cytoplasmic hydrogen index (pH) [18], and an increase in the concentration of intracellular potassium ([K+]i) and sodium ([Na+]i) [19]. The authors [19] attribute this only to an increase in HIV-1 syncytium-forming activity, which at the same time does not lead to an increase in virus yield. But it should be noted that, according to their data, in a chronically HIV-1-infected neoplastic CD4+ T cell line, in which there is virtually no cytopathic effect, potassium levels are significantly higher than in control uninfected cells. This indicates that HIV-1 replication increases cellular potassium levels and this may be a factor necessary for viral replication.
The reason for increased HIV-1 replication may also be due to differences in the interaction mechanisms of other intracellular components that may influence the infection process. As shown by cytometric studies of cell lines, in addition to CD38 protein, expression of CD28 protein was enhanced in the high-yielding line. In 72 h after cell reseeding we observed a 3.2-fold increase in the number of CD28+ cells in it [11]. This costimulatory surface protein molecule plays a key role in the activation and regulation of CD4+ T-cell function [20]. Over the past decade, the use of advanced technologies has provided a wealth of data on the host factors that viruses use to spread infection, as well as on the antiviral factors responsible for suppressing viral infection. In the pathogenesis of adult T-cell leukemia/lymphoma, it appears that the functional activity of this protein may be one of the components regulating the features of the oncogenic process. Multiple alterations in the gene of this protein and changes in the chain of sequential activating signals in which this protein participates have been studied [21]. Its activation affects signaling pathways involved in the regulation of interleukin-2 (IL-2) expression by cells. IL-2 regulates the activity of inducible T-cell kinase (ITK, a family of cytoplasmic TEC-tyrosine kinases) [22]. ITC has been shown to facilitate HIV-1 replication, and the enzyme is required for efficient virus entry, reverse transcription, assembly and release of viral particles [23]. Thus, the enhanced HIV-1 activity in the MT-4/2 cell line can also be explained by increased expression of CD28 protein and the associated increase in ITC activity.
Using two variants of the MT-4 cell line with different replicative activity allowed us to perform a comparative analysis of the antiviral efficacy of NRTI 6NR and 3TS on HIV-1 depending on the viral load. Our studies (Fig. 2) showed that the degree of inhibition of viral activity by the drugs individually was lower in the MT-4/2 line. Moreover, the antiviral effect of 3TS was more strongly dependent on viral activity than 6NR. When the drugs were used together, a high antiviral effect was observed and was independent of the level of viral replication. High antiviral activity was found when lower concentrations of the substances were used together than when they were used separately. When the drugs were used in combination, the antiviral effect was almost similar in all cases and ranged from 87‒96% for the MT-4/1 line and 83‒89% for the MT-4/2 line. It cannot be excluded that the metabolism of 6HP and 3TC in the cells of these lines may have its own peculiarities and their identification in the future will allow a more accurate assessment of the efficacy of the tested drugs.
Conclusion
The results obtained indicate that in the experimental in vitro HIV-1/MT-4 cell system, when NRTI 6NR and 3TS were used individually, a reduced antiviral effect was observed in the system with higher viral activity. Co-administration of the drugs showed a high level of inhibition when lower doses of the drugs were used, regardless of viral replicative activity. The combined use of these drugs is promising for the treatment of HIV-infected patients at different stages of infection and with different levels of viral load.
1 Joint United Nations Programme on HIV/AIDS (UNAIDS). Available at: https://unaids.org
About the authors
Lyudmila B. Kalnina
The D.I. Ivanovsky Research Institute of Virology the N.F. Gamaleya NRCEM of the Ministry of Health of the Russian Federation
Email: klb3@yandex.ru
ORCID iD: 0000-0002-2702-8578
Candidate of Biological Sciences, Leading researcher of the Laboratory of Antiviral and Disinfection Agents
Russian Federation, 123098, MoscowLyudmila M. Selimova
The D.I. Ivanovsky Research Institute of Virology the N.F. Gamaleya NRCEM of the Ministry of Health of the Russian Federation
Author for correspondence.
Email: lselim@mail.ru
ORCID iD: 0000-0003-3709-770X
Doctor of Biological Sciences, Leading researcher of the Laboratory of Antiviral and Disinfection Agents
Russian Federation, 123098, MoscowDmitry N. Nosik
The D.I. Ivanovsky Research Institute of Virology the N.F. Gamaleya NRCEM of the Ministry of Health of the Russian Federation
Email: dnnosik@yandex.ru
ORCID iD: 0000-0001-5757-5671
Professor, Doctor of Medical Sciences, Head of the Laboratory of Antiviral and Disinfection Agents
Russian Federation, 123098, MoscowReferences
- Kireev D.E., KirichenkoA.A., Lopatukhin A.E., Shlykova A.V., Galkin N.Yu, Saveler E.V et al. The Russian database of HIV antiretroviral drug resistance. Journal of Microbiology, Epidemiology and Immunobiology. 2023,100(2):219-227. DOI: https://doi.org/10.36233/0372-9311-345
- Ndung’u T., McCune J.M., Deeks S.G. Why and where an HIV cure is needed and how it might be achieved. Nature. 2019; 576(7787): 397–405. https://doi.org/10.1038/s41586-019-1841-8
- Menéndez-Arias L., Sebastián-Martín A., Álvarez M. Viral reverse transcriptases. Virus Res. 2017; 234: 153–76. https://doi.org/10.1016/j.virusres.2016.12.019
- Mitsuya H., Weinhold K.J., Furman P.A., St Clair M.H., Lehrman S.N., Gallo R.C., et al. 3’-azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl Acad. Sci. USA. 1985; 82(20): 7096–100. https://doi.org/10.1073/pnas.82.20.7096
- Menéndez-Arias L. Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res. 2013; 98(1): 93–120. https://doi.org/10.1016/j.antiviral.2013.01.007.
- Khandazhinskaya А.L., Shirokova E.A. AZT 5’-Phosphonates: achievements and trends in the treatment and prevention of Hiv infection. Acta Naturae. 2013; 5(3): 54–61. https://elibrary.ru/rvzyvv
- Galegov G.A.1, Andronova V.L. AntiHIV/AIDS drug 6HP: antiviral activity, pre-clinical study. Efficiency in adult HIV-infected patients. Voprosy virusologii. 2019; 64(1): 12–5. https://doi.org/10.18821/0507-4088-2019-64-1-12-15 https://elibrary.ru/yzkhjj (in Russian)
- Quercia R., Perno C.F., Koteff J., Moore K., McCoig C., St Clair M., et al. Twenty-five years of lamivudine: current and future use for the treatment of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2018; 78(2): 125–35. https://doi.org/ 10.1097/QAI.0000000000001660.
- Perry C.M., Faulds D. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs. 1997; 53(4): 657–80. https://doi.org/10.2165/00003495-199753040-00008
- Ma A., Chen D.M., Chau F.M., Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010; 22(10): 1189–94. https://doi.org/10.1080/09540121003668102
- Nosik D.N., Kalnina L.B., Selimova L.M., Pronin A.V. An increase in the infectivity of the human immunodeficiency virus with modification of the CCR5 gene receptor of sensitive cells. Doklady Rossiiskoi Akademii nauk. Nauki o zhizni. 2023; 511(1): 344–8. https://doi.org/10.31857/S2686738923700257 https://elibrary.ru/jiltbd (in Russian)
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983; 65(1-2): 55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
- Manns A., Hisada M., La Grenada L. Human T-lymphotropic virus type 1 infection. Lancet. 1999; 353(9): 1951–8. https://doi.org/10.1016/s0140-6736(98)09460-4
- Selimova L.M., Kalnina L.B., Nosik D.N. The superficial markers of neoplastic cell line MT-4 and perspectives of its application as a model for studying activity of immune modulating preparations. Klinicheskaya laboratornaya diagnostika. 2016; 61(12): 822–5. https://doi.org/10.18821/0869-2084-2016-61-12-822-825 https://elibrary.ru/xscfqz (in Russian)
- Morandi F., Airoldi I., Marimpietri D., Bracci C., Faini A.C., Gramignoli R. CD38, a receptor with multifunctional activities: from modulatory functions on regulatory cell subsets and extracellular vesicles, to a target for therapeutic strategies. Cells. 2019; 8(12): 1527–44. https://doi.org/10.3390/cells8121527
- Dimitrov D.S., Broder C.C., Berger E.A., Blumenthal R. Calcium ions are required for cell fusion mediated by the CD4-human immunodeficiency virus type 1 envelope glycoprotein interaction. J. Virol. 1993; 67(3): 1647–52. https://doi.org/10.1128/JVI.67.3.1647-1652.1993
- Cloyd M.W., Lynn W.S. Perturbation of host-cell membrane is a primary mechanism of HIV cytopathology. Virology. 1991; 181(2): 500–11. https://doi.org/10.1016/0042-6822(91)90882-c
- Makutonina A., Voss T.G., Plymale D.R., Fermin C.D., Norris C.H., Vigh S., et al. Human immunodeficiency virus infection of T-lymphoblastoid cells reduces intracellular pH. J. Virol. 1996; 70(10): 7049–55. https://doi.org/10.1128/JVI.70.10.7049-7055.1996
- Voss T.G., Fermin C.D., Levy J.A., Vigh S., Choi B., Garry R.F. Alteration of intracellular potassium and sodium concentrations with induction of cytopathic effects by human immunodeficiency virus. J. Virol. 1996; 70(8): 5447–54. https://doi.org/10.1128/JVI.70.8.5447-5454.1996
- Esensten J.H., Helou Y.A., Chopra G., Weiss A., Bluestone J.A. CD28 costimulation: from mechanism to therapy. Immunity. 2016; 44(5): 973–88. https://doi.org/10.1016/j.immuni.2016.04.020
- Sakamoto Y., Ishida T., Masaki A., Takeshita M., Iwasaki H., Yonekura K., et al. Clinical significance of CD28 gene-related activating alterations in adult T-cell leukaemia/lymphoma. Br. J. Haematol. 2021; 192(2): 281–91. https://doi.org/10.1111/bjh.17211
- Lechner K.S., Neurath M.F., Weigmann B. Role of the IL-2 inducible tyrosine kinase ITK and its inhibitors in disease pathogenesis. J. Mol. Med. (Berl.). 2020; 98(10): 1385–95. https://doi.org/10.1007/s00109-020-01958-z.
- Readinger J.A., Schiralli G.M., Jiang J.K., Thomas C.J., Avery A., Henderson A.J., et al. Selective targeting of ITK blocks multiple steps of HIV replication. Proc. Natl Acad. Sci. USA. 2008; 105(18): 6684–9. https://doi.org/10.1073/pnas.0709659105
Supplementary files