Characteristics of imported cases of Dengue fever and hemorrhagic Dengue fever in 2009–2019
- Authors: Sayfullin M.A.1,2, Zvereva N.N.1, Karan L.S.3, Grigoreva Y.E.3, Akinshina Y.A.2,4, Larichev V.F.2, Shamsheva O.V.1, Bazarova M.V.5, Smetanina S.V.5
-
Affiliations:
- Pirogov Russian National Research Medical University
- Research Center for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya of the Ministry of Healthcare of the Russian Federation
- Central Research Institute of Epidemiology» of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing
- CJSC “EcoLab”
- City Infectious Clinical Hospital No. 1, Moscow
- Issue: Vol 67, No 4 (2022)
- Pages: 322-330
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/634
- DOI: https://doi.org/10.36233/0507-4088-126
- ID: 634
Cite item
Abstract
Introduction. In Russia, the approved morbidity statistics system is represented by the International Classification of Diseases of the 10th revision (ICD-10). This classification provides two forms of dengue fever (DF): dengue fever (A90) and hemorrhagic dengue (A91). Official statistics on the ratio of forms of DF is not published in open sources and this lack of information about the real ratio of the forms of DF makes it difficult to objectively assess the factors that determine the severity of this disease.
The aim: compare the clinical and epidemiological features of dengue fever and hemorrhagic dengue fever in patients hospitalized in 2009–2019 to the City Infectious Clinical Hospital No. 1, Moscow.
Materials and methods. A retrospective cohort study. We analyzed the patient database and reviewed 391 medical records of patients with diagnosed dengue fever. We compared gender, age characteristics, travel geography including information about previous visits of patients to endemic regions and dengue virus serotype. To determine the primary and re-infection rate, an analysis of IgG for the dengue virus was carried out on days 1–5 of the disease. To compare indicators, 95% confidence intervals for proportions, medians, and interquartile ranges were calculated. The significance of differences between independent samples for assessing qualitative characteristics was carried out using the criteria χ2, the odds ratio. To assess the quantitative characteristics, the Mann-Whitney test was used. Differences were considered statistically significant at p ≤ 0.05.
Results. The proportion of patients with dengue fever was 14.9% of all hospitalized with febrile illnesses that developed after international travel. Hemorrhagic dengue fever (DHF) was diagnosed in 15.7% of patients with dengue fever. DHF developed significantly more often in women, as well as in those who had history of repeated visits to endemic regions. However, DHF was also diagnosed in 10.9% of first-time travelers to tropical countries. We did not find significant differences in the rates of DHF development depending on age and dengue virus serotype. In a number of patients who had not previously traveled to endemic regions, IgG to the dengue virus were detected, which may indicate a previous infection with related flaviviruses.
Conclusion. It has been established that in the regions most visited by Russians, there is a circulation of all serotypes of the dengue virus with an annual change in the predominant serotype.
Keywords
Full Text
Introduction
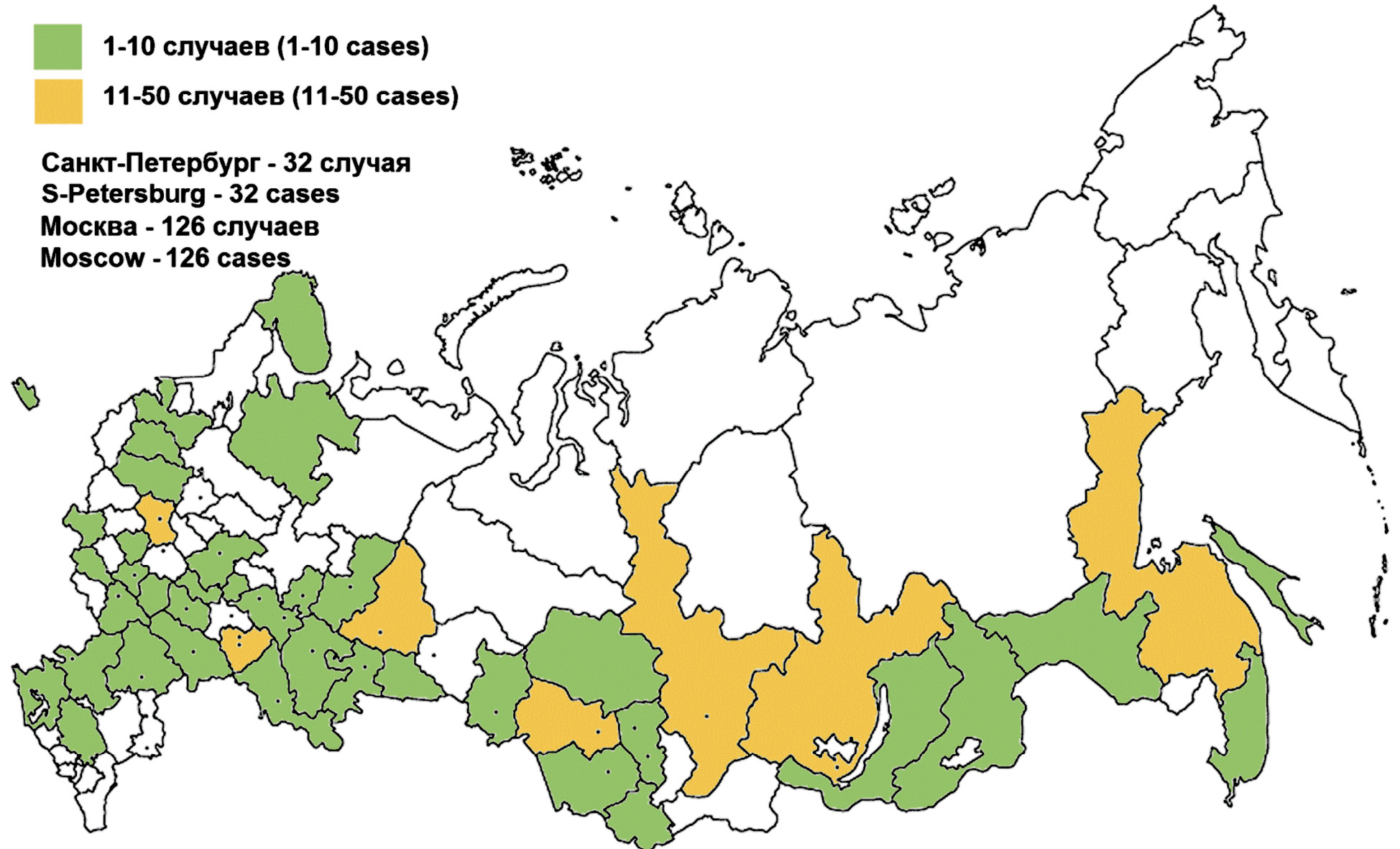
The first imported case of dengue fever (DF) in Russia was reported in 2002 [1]. A few isolated imported cases were confirmed during the next 10 years. After the DF record-keeping had been standardized in 2012, a total of 1,618 dengue cases were reported over 9 years. In 2019, 415 dengue cases were reported in 48 of 85 territorial entities of the Russian Federation, including 126 (30.4%) in Moscow [2] (Fig. 1).
Fig. 1. Distribution of cases of dengue fever in the Russian Federation in 2019 (according Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing).
In Russia, the approved morbidity statistics system1 is represented by the International Classification of Diseases, Tenth Revision (ICD-10), which classifies dengue into two major categories: classic (DF, A90) and hemorrhagic (DHF, A91) dengue fever. No data are available on the ratio between the above forms of the disease in the public statistics sources. Information about DHF cases in Russia, including two cases with fatal outcomes in Moscow and Novosibirsk, is available in scientific publications [3–7]. The lack of information about the actual ratio of DF forms makes it difficult to assess the factors contributing to the severity of the infection. The standard tourniquet test [5, 7] recommended by the World Health Organization (WHO) for DF diagnosis is not always used or is replaced with non-standardized provocative tests [7, 8].
The DHF development is usually associated with the antibody-dependent enhancement (ADE) of infection, which, according to S. Halstead, occurs during secondary heterologous dengue virus infection or primary infection in people who passively acquired specific antibodies, including infants born to seropositive mothers [9, 10]. The cases of reinfection with the homologous dengue virus, which are described in literature [11], do not look convincing and call for further research. The concept of DHF development during reinfection is included in the clinical practice guidelines “Dengue Fever in Adults”, which were published in 2014 [12]. Currently, increasing attention is given to other factors of DHF development: specific DENV serotypes [13–15], gender, race, genetic characteristics [16–18]. Therefore, severe forms of the disease can depend on different factors, which may cause high levels of viremia, release of inflammatory cytokines, damage to endothelial cells, thus, affecting the disease severity and prognosis [10, 19, 20].
The aim of our study was to analyze clinical and epidemiological characteristics of DF and DHF in patients hospitalized to Infectious Disease Clinical Hospital No. 1 (IDCH No. 1) in Moscow in 2009–2019.
Materials and methods
Study design: retrospective cohort study.
Materials: The patient database (created by the authors using MS Access). Medical records of the patients hospitalized to IDCH No. 1 from 2009 to 2019.
Inclusion criteria: Medical records of the patients who were hospitalized to IDCH No. 1 and developed the disease within 21 days after they had left the country for international travel. This group was further screened to select medical records of patients with the laboratory-confirmed diagnosis of dengue fever. The study was performed with the informed consent of patients2. The research protocol was approved by the Ethics Committee of the The D.I. Ivanovskiy Institute of Virology, protocol №1 dated 20.03.2014.
Specific diagnostic techniques: Dengue virus-specific IgM antibodies were detected using the ELISA-IgM Zika, dengue, WN, Chik reagent kit registered in Russia and intended for differential detection of IgM antibodies to Zika, dengue, West Nile and chikungunya viruses in human sera by the enzyme-linked immunosorbent assay (ELISA). The testing system is used to detect group-specific antibodies to all the four serotypes of the dengue virus. Dengue virus-specific IgG antibodies were detected using the ELISA-IgG pilot kit developed and made by the Laboratory of Arbovirus Biology and Indication at the Gamaleya National Research Center for Epidemiology and Microbiology (NRCEM).
The ribonucleic acid of the dengue virus was detected and the type of the virus was identified using AmpliSens Dengue virus-FL and AmpliSens Dengue virus type-Fl pilot and subsequently licensed reagent kits (Central Research Institute of Epidemiology, Rospotrebnadzor, Moscow).
The DHF diagnosis was made in accordance with the criteria established by WHO in 1997 [20]. Other important details included the age of patients, their gender, visits to dengue-endemic regions, mosquito contact, existence of specific IgG antibodies during the first 5 days of the illness onset, results of dengue virus genotyping.
The statistical analysis was performed using MS Excel and IBM SPSS Statistics 23.0. Bootstrap distribution of proportions was used to calculate 95% confidence intervals (CI). The data distribution was analyzed with Shapiro–Wilk and Kolmogorov–Smirnov tests. Median values and interquartile ranges were calculated for non-parametric variables. The χ2 test was used to assess the significance of differences between independent samples; the odds ratio (OR) was used to assess categorical variables; the Mann–Whitney U test and the Kruskal–Wallis test were used for assessment of quantitative variables. Differences were considered statistically significant at p ≤ 0.05.
Results
General profile of patients
the study was based on medical records of patients hospitalized to IDCH No. 1 during 2009–2019; the selected group included 2,632 patients with febrile illnesses developed within 21 days after they had visited other countries. Among them, 391 patients (14.9%) were diagnosed with dengue fever, including 330 patients (84.3%) with DF, 57 patients (14.6%) with grade I–II DHF, and 4 patients (1.1%) with grade III–IV DHF. Among the dengue patients, there were 208 (53.2%) women.
During the previous 10 years, the number of imported dengue cases was stable, ranging from 20 to 40 a year; however, in 2019, a significant increase in the number of cases was reported, having been presumably associated with the increased incidence in countries of Southeast Asia as well as with the number of international trips, which increased by 18.9% compared to the previous years3. During different years of observation, the ratio between the number of DHF and DF cases ranged from 0 to 33.3%, with DHF having been detected in 15.7% of the total number of patients (Fig. 2).
Fig. 2. The number of cases of dengue fever diagnosed in city Infectious Clinical Hospital (Moscow) in 2009–2019.
The age of the patients ranged from 1 to 65 years, with the median age of 30 years [26; 38]; there were 16 (4.1%) children aged 1–18 years, including one 9-year-old girl diagnosed with DHF. The median age of DF patients was 30 years [26; 37]; the median age of DHF patients – 31 years [26; 38] (pU > 0,05). One child was diagnosed with DHF. The differences in DHF frequency between children and adults were not analyzed statistically due to few observations in the pediatric group.
Female patients dominated significantly among the DHF patients – 43 women (70.5%; 95% CI, 58.7–81.4), while in the DF group there were 165 women (50%; 95% CI, 43.8–54.5), pχ2 = 0.003, OR = 2.45 (95% CI, 1.4–4.4).
Dengue patients were admitted to hospital during the entire year, much more frequently in January (15.3%) and less frequently in October (3.8%).
113 (28.4%) patients were hospitalized on the 1st–3rd day of the disease; 215 (55%) – on the 4th–6th day; 52 (13.3%) on the 7th–9th day; 11 (2.8%) – on the 10th day and later after the onset of disease symptoms.
Association between infection cases and visits to endemic regions
We have found the causative relationship between the disease and trips to 23 tropical countries: 373 trips (95.4%) were to Southeast and South Asia, including Thailand – 223 (57%), Indonesia – 68 (17.4%), Vietnam – 36 (9.2%), the Maldives – 15 (3.8%), and India – 13 (3.3%). In countries of the Western hemisphere, a total of 12 people (3.1%) were infected, including 7 – in the Dominican Republic and 6 (1.5%) – in African countries.
277 (70.8%) patients informed about mosquito bites during the trip; 60 (15.3%) patients denied their contact with any insects; 54 (13.8%) patients mentioned the presence of mosquitoes, but they were not sure whether mosquitoes had bitten them.
The length of stay in a dengue-endemic region was known in 360 cases and ranged from 3 days to 2 years: 86 (23.8%) patients stayed there from 3 to 10 days; 234 (65%) patients – from 11 to 20 days; 26 (7.2%) patients – from 21 to 30 days; 14 (3.9%) patients stayed in endemic areas longer or lived there permanently; the median length of stay in an endemic region was 15 days [11; 16].
Among the patients, 382 (97.6%) were Russian citizens, 155 (39.6%) visited tropical regions for the first time, 161 (41.1%) had already been there, including citizens of India (2), Vietnam (4), Angola, Peru, Thailand (one person from each country). Two citizens of Vietnam informed that they had dengue fever previously. 75 patients (19.2%) did not provide any anamnestic data on their trips, which would be useful for the analysis.
After their first visit to endemic regions, 18 patients of 173 (10.9%) developed DHF; after repeat trips, 35 patients of 161 (22.3%) developed DHF; pχ = 0.007, OR = 2.34 (95% CI, 1.26–4.8) (Table 1).
Table 1. The frequency of development of DF and DHF depending on the frequency of visits to endemic regions
Таблица 1. Частота развития классической и геморрагической формы лихорадки денге в зависимости от кратности посещения эндемичных регионов
Diagnosis Диагноз | Visit count (%; 95% CI) Количество (%; 95% ДИ) | |
First visit Первичное посещение | Return visit Повторное посещение | |
Dengue fever (DF) Классическая | 138 (89.1%; 84.3–93.7) | 126 (77.6%; 71.3–83.6) |
Dengue Hemorrhagic fever (DHF) Геморрагическая | 17 (10.9%; 6.3–15.7) | 35 (22.4%; 16.4–28.7) |
The longest incubation period was 14–15 days (based on the onset of the first symptoms after the patients came back to Russia); we were unable to identify the shortest and average incubation time due to absence of the confirmed relationship between mosquito bites and the onset of the disease.
Levels of specific IgM and IgG antibodies in dengue patients
Using IgM and IgG ELISA, together with the tetravalent dengue virus antigen, we tested 26 sera collected on the 2nd–5th day of the disease (from 22 DF patients and from 4 DHF patients). All the samples were tested positive for IgM antibodies with titers 1 : 100 to 1 : 6,400. IgG antibodies were detected in 9 patients (34.6%): 3 DHF patients and 6 DF patients (27.3%). Out of them, 5 patients (3 – with DHF, 2 – with DF) did not have any information evidencing that they had ever visited endemic regions (Table 2). Out of 17 IgG-negative patients, only one woman who had visited endemic regions developed DHF.
Table 2. The value of the factor of the presence of specific IgG antibodies in the early period of the disease (2–5 days)
Таблица 2. Значение фактора наличия специфических IgG-антител в ранний период заболевания (2–5 суток)
Gender, Age Пол, возраст | Return visit in endemic region Повторное посещение эндемичного региона | Diagnosis Форма заболевания | Diseases day День болезни | Reciprocal value of the titer Обратная величина титра | |
IgG | IgM | ||||
F (Ж), 36 | Yes (Да) | DF (КЛД) | 2 | 1600 | 200 |
М (M), 41 | Yes (Да) | DF (КЛД) | 3 | 100 | 100 |
F (Ж), 29 | No (Нет) | DHF (ГЛД) | 4 | 100 | 100 |
F (Ж), 26 | No (Нет) | DF (КЛД) | 5 | 200 | 100 |
F (Ж), 20 | No (Нет) | DF (КЛД) | 5 | 3200 | 100 |
F (Ж), 27 | No (Нет) | DHF (ГЛД) | 5 | 200 | 800 |
М (M), 31 | Yes (Да) | DF (КЛД) | 5 | 12 800 | 800 |
F (Ж), 23 | No (Нет) | DHF (ГЛД) | 5 | 100 | 1600 |
М (M), 30 | Yes (Да) | DF (КЛД) | 5 | 100 | 6400 |
Relationship between the disease form and the virus serotype
Plasma samples collected from 246 patients were tested using the reverse transcription polymerase chain reaction (RT-PCR), and the virus serotype was identified in samples from 244 patients: DENV-1 – 121 (49.5%), DENV-2 – 77 (31.5%), DENV-3 – 30 (12.3%), DENV-4 – 15 (6.1%); one patient had coinfection with DENV-1 and DENV-2. Two patients, who were hospitalized on the 4th day (DHF) and 8th day (DF) of the disease, had a negative PCR test result. During different years, the ratio between the variants varied significantly, demonstrating the dominance of DENV-1 or DENV-2.
Each of the dengue virus serotypes caused both forms of the disease, though the proportions were different: DENV-3 accounted for the largest proportion of DHF (Table 3); no statistically significant differences in DHF proportions in patients with different virus serotypes have been found (pkw = 0.1). No etiological significance of dengue viruses in development of primary and secondary dengue cases has been found.
Table 3. The ratio of cases of DF and DHF infected of different DENV serotypes (count, percent, 95%CI)
Таблица 3. Соотношение случаев классической и геморрагической денге при инфицировании различными серотипами
Diagnosis Диагноз | Case count (%, 95% CI) Количество случаев (%, 95% ДИ) | |||
DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
Dengue fever (DF) Классическая | 19 (15,6%; 9,7–21,9) | 10 (12,1%; 5,5–20,6) | 9 (30,0%; 14,8–48,1 | 1 (6,7%; 0–22,7) |
Hemorrhagic Dengue fever (DHF) Геморрагическая | 103 (84,4%; 78,1–90,3) | 68 (87,9%; 79,4–89,5) | 21 (70%; 51,9–85,2) | 14 (93,3%; 77,3–100) |
The ratio between disease forms in patients who visited hyperendemic regions of Southeast Asia
DHF was diagnosed after trips to 9 countries, including 31 cases (51%) after the trip to Thailand, 12 cases (19.6%) – to Indonesia, 7 cases (11.5%) – to Vietnam, 5 (8.2%) – to the Maldives. Rare cases were imported India, Myanmar, Peru, and the Dominican Republic. Thus, 96.7% of DHF cases were reported after trips to countries of South and Southeast Asia. The DHF proportion in countries with 10 and more cases ranged from 13.9% (95% CI, 9.5–18.9) in Thailand to 19.4% (95% CI, 7.5–33.3) in Vietnam. In addition, DHF was diagnosed in two citizens of Vietnam, who had DF previously. No statistically significant differences in the ratio between the disease forms among the countries with the number of cases exceeding 10 people have been found (pχ2 = 0.221).
The etiological role of the DENV-1 serotype was identified in 49.5% of cases, while in different hyperendemic countries, its proportion ranged from 33.3% to 68.0%; all the 4 serotypes in different ratios were detected in countries with 10 and more hospitalized patients (Fig. 3).
Fig. 3. The ratio of dengue virus serotypes in patients arriving from Thailand, Indonesia and Vietnam.
The long-term monitoring of hospitalized patients who returned from Thailand showed that the dominance of serotypes from the same region was not permanent: The significant dominance of DENV-1 circulation was observed in 2013 and 2019, while DENV-3 prevailed in 2014 and DENV-2 was dominant in 2018 (Fig. 4).
Fig. 4. The ratio of dengue virus serotypes in patients infected in Thailand 2013–2019.
Discussion
before the COVID-19 pandemic, Russia demonstrated a steady upward trend in verified imported dengue cases. Over 10 years of observation, the number of dengue virus infection cases increased from a few isolated cases to the numbers exceeding the counts of cases of natural-focal infections endemic for Russia. The increase in dengue incidence is associated with increased international travel and with development of specific diagnostic techniques. Unlike cases in endemic regions, dengue cases in Russia are imported and, consequently, have their specific characteristics such as seasonality, age composition, a significantly smaller proportion of affected children, comparatively rare cases of unfavorable disease progression (two deaths over the entire observation period). As it is known, the primary infection with one of the four dengue viruses can usually lead to development of the classical form of the disease. However, we observed a fatal outcome after the first visit to the dengue-endemic region [2].
Based on our data, the repeat visit to an endemic region increases the risk of DHF development, which in most cases is characterized by the decreased platelet count, a positive tourniquet test and (or) spontaneous minor bleeding. The pathogenic significance of the antibody-dependent enhancement of infection in DHF cases is supported by high rates of IgG detection in patients during the early stage of the disease. However, further studies are required to make any statistically sound conclusions in this respect. Nevertheless, the absence of IgG in one patient with DHF during the early stage of the disease can be indicative of the primary infection. During early stages of the disease, the presence of group-specific IgG in patients who did not visit endemic regions can be associated with the previous infection with related flaviviruses (the West Nile virus, tick-borne encephalitis, etc.) or with vaccination against tick-borne encephalitis.
We have not found any relationship between the form of the disease and the specific DENV serotype, though we found it out that all the four serotypes circulated in hyperendemic countries, which are highly popular among Russian tourists. In addition, it has been found that in dengue-endemic regions, the ratios of circulating dengue viruses are constantly changing, thus increasing the risk of repeat infection with the heterologous virus during the re-visit to the same geographic area. Therefore, prior to their next trip, the individuals who recovered from dengue fever must be informed about potential risks, required preventive measures and the need to seek immediate medical care in case of any febrile illness that occurred during the trip or within two weeks after the trip to endemic regions.
Conclusions
It has been found that women are at higher risk of developing the hemorrhagic form of dengue; these findings are consistent with the findings of the previous studies.
Repeat visits to endemic regions increase the risk of DHF development, though this form of the disease can be observed in individuals after their first visit to endemic tropical countries.
Heterologous IgG detected during early stages of the disease in individuals who did not visit dengue-endemic regions may result from the previous infection with related viruses or from vaccination.
Limitations of the study
Considering that the study was retrospective and that we analyzed documented data, the time of collection of clinical material from hospitalized patients was not sufficiently standardized. The amount of the biological material collected from some patients during the first days of the disease was limited due to their late admission and hospitalization.
1Decree of the Ministry of Health of the Russian Federation, No. 170, May 27, 1997, “On Transition of RF Healthcare Authorities and Institutions to the International Statistical Classification of Diseases and Health-Related Conditions, 10th Revision”.
2The standard consent form for minimally invasive manipulations, collection of biological material and personal data analysis was filled out upon admission of the patient to hospital.
3Based on the data from the Russian Federal Agency for Tourism, the number of international trips was 119% in 2019 compared to the average level in 2014–2018.
About the authors
Mukhammad A. Sayfullin
Pirogov Russian National Research Medical University; Research Center for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya of the Ministry of Healthcare of the Russian Federation
Author for correspondence.
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0003-1058-3193
Russian Federation, 117997, Moscow; 123098, Moscow
Nadezda N. Zvereva
Pirogov Russian National Research Medical University
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0003-2699-0439
Russian Federation, 117997, Moscow
Luidmila S. Karan
Central Research Institute of Epidemiology» of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0002-5927-460X
Russian Federation, 111123, Moscow
Yana E. Grigoreva
Central Research Institute of Epidemiology» of the Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0001-9016-9923
Russian Federation, 111123, Moscow
Yulia A. Akinshina
Research Center for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya of the Ministry of Healthcare of the Russian Federation; CJSC “EcoLab”
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0002-9223-3455
Russian Federation, 123098, Moscow; 142530, Elektrogorsk, Moscow region
Victor F. Larichev
Research Center for Epidemiology and Microbiology named after the honorary academician N.F. Gamaleya of the Ministry of Healthcare of the Russian Federation
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0001-8262-5650
Russian Federation, 123098, Moscow
Olga V. Shamsheva
Pirogov Russian National Research Medical University
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0002-6033-6695
Russian Federation, 117997, Moscow
Marina V. Bazarova
City Infectious Clinical Hospital No. 1, Moscow
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0001-7322-7896
Russian Federation, 125367, Moscow
Svetlana V. Smetanina
City Infectious Clinical Hospital No. 1, Moscow
Email: dr_saifullin@mail.ru
ORCID iD: 0000-0003-3763-697X
Russian Federation, 125367, Moscow
References
- Larichev V.F., Sayfullin M.A., Akinshina Yu.A., Khutoretskaya N.V., Butenko A.M. Imported cases of arbovirus infections in the Russian Federation. Epidemiologiya i infektsionnye bolezni. 2012; (1): 35–8. (in Russian)
- State report «On the state of sanitary and epidemiological welfare of the population in the Russian Federation in 2020». Moscow; 2021. (in Russian)
- Sayfullin M.A., Kelli E.I., Bazarova M.V., Larichev V.F., Karan’ L.S., Akinshina Yu.A., et al. Dengue fever fatal case. Epidemiologiya i infektsionnye bolezni. 2015; 20(2): 49–51. (in Russian)
- D’yachkov A.G., Lioznov D.A., Doroshkevich V.V. Case report of dengue hemorrhagic fever. Zhurnal infektologii. 2013; 5(3): 71–3. https://doi.org/10.22625/2072-6732-2013-5-3-71-73 (in Russian)
- Safonov A.D. A case of imported dengue hemorrhagic fever in Omsk. Epidemiologiya i infektsionnye bolezni. 2012; (1): 49–51. (in Russian)
- Nadeev A.P., Mal’tseva Yu.G., Shishkina E.Yu., Porotnikova E.V., Khokhlova N.I. Fatal dengue fever. Arkhiv patologii. 2020; 82(1): 52–5. https://doi.org/10.17116/patol20208201152 (in Russian)
- Nechaev V.V., Yarovaya I.I., Kachenya G.V., Doguzhieva E.V., Buntovskaya S.S., Egorikhina A.D., et al. Clinical-epidemiological characteristics delivery cases of tropic dengue fever. Zhurnal infektologii. 2021; 13(1): 78–85. https://doi.org/10.22625/2072-6732-2021-13-1-78-85 (in Russian)
- Khokhlova N.I., Krasnova E.I., Pozdnyakova L.L. Clinical and laboratory diagnosis of dengue fever in travelers. Zhurnal infektologii. 2015; 7(1): 65–9. https://doi.org/10.22625/2072-6732-2015-7-1-65-69 (in Russian)
- WHO. Fact sheets. Dengue and severe dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- Halstead S. Recent advances in understanding dengue. F1000Res. 2019; 8: F1000 Faculty Rev-1279. https://doi.org/10.12688/f1000research.19197.1
- Waggoner J.J., Balmaseda A., Gresh L., Sahoo M.K., Montoya M., Wang E., et al. Homotypic dengue virus reinfections in Nicaraguan children. J. Infect. Dis. 2016; 214(7): 986–93. https://doi.org/10.1093/infdis/jiw099
- Dengue fever in adults. Clinical recommendations. Approved by the decision of the Plenum of the Board of the National Scientific Society of Infectious Diseases on October 30, 2014. Available at: https://nnoi.ru/uploads/files/protokoly/Lih_Denge_adult.pdf (in Russian)
- Holmes E.C., Twiddy S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003; 3(1): 19–28. https://doi.org/10.1016/s1567-1348(03)00004-2
- Simmons C.P., Farrar J.J., Nguyen V.C., Wills B. Dengue. N. Engl. J. Med. 2012; 366(15): 1423–32. https://doi.org/10.1056/NEJMra1110265
- Huy N.T., Van Giang T., Thuy D.H., Kikuchi M., Hien T.T., Zamora J., et al. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013; 7(9): 2412. https://doi.org/10.1371/journal.pntd.0002412
- Nikolaeva L.I., Larichev V.F., Sayfullin M.A., Dedova A.V., Grishechkin A.E., Vasil’ev A.V., et al. Analysis of the possible association of patients’ genetic factors with course of dengue virus infection. Infektsionnye bolezni: Novosti. Mneniya. Obuchenie. 2022; 11(1): 8–14. https://doi.org/10.33029/2305-3496-2022-11-1-8-14 (in Russian)
- Bhatt P., Sabeena S.P., Varma M., Arunkumar G. Current understanding of the pathogenesis of Dengue virus infection. Curr. Microbiol. 2021; 78(1): 17–32. https://doi.org/10.1007/s00284-020-02284-w
- Xavier-Carvalho C., Cardoso C.C., de Souza Kehdy F., Pacheco A.G., Moraes M.O. Host genetics and dengue fever. Infect. Genet. Evol. 2017; 56: 99–110. https://doi.org/10.1016/j.meegid.2017.11.009
- WHO. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. Geneva; 1997.
- Harapan H., Michie A., Sasmono R.T., Imrie A. Dengue: a minireview. Viruses. 2020; 12(8): 829. https://doi.org/10.3390/v12080829
Supplementary files