Recombinant VP1 protein of norovirus GII.4 (Caliciviridae: Norovirus) is capable to induse the production of cross-reacting antibodies
- Authors: Lapin V.A.1, Novikov D.V.1, Kashnikov A.Y.1, Epifanova N.V.1, Novikova N.A.1, Mokhonova E.V.1, Melentev D.A.1, Tsyganova M.I.1, Zaitsev D.E.1, Novikov V.V.1
-
Affiliations:
- Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
- Issue: Vol 70, No 3 (2025)
- Pages: 282-290
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16753
- DOI: https://doi.org/10.36233/0507-4088-316
- EDN: https://elibrary.ru/HHCKYL
- ID: 16753
Cite item
Abstract
Introduction. Norovirus (NoV) is one of the main causes of acute gastroenteritis. Currently, there is no vaccine to prevent norovirus infection. Vaccines under development are based on the capsid protein VP1, which is capable of forming virus-like particles.
The aim of the work was to analyze the immunogenic properties of the recombinant VP1 protein of NoV GII.4.
Materials and methods. In the blood serum of animals immunized with the recombinant VP1 protein obtained by the authors, titers and avidity of total antibodies and IgM antibodies against NoV VP1 were determined using enzyme immunoassay. The ability of the obtained antibodies to interact with NoV of different genotypes was assessed using immunoelectron microscopy.
Results. The recombinant VP1 protein induced high titer antibody production in animals. Total antibodies against VP1 had a high avidity, reaching 100%, which suggests that they have viral neutralizing activity. IgM antibodies had low avidity. Immunoelectron microscopy showed that IgG antibodies against VP1 protein of genotype GII.4 interact with wild-type NoV of genotype GII.7 and GII.17.
Conclusion. The obtained recombinant protein induces a sufficiently strong immune response with the formation of high avidity polyclonal cross-reacting antibodies, which allows us to consider it as an antigen component of a NoV vaccine candidate.
Full Text
Introduction
Noroviruses (NoV) are the second leading cause of acute gastroenteritis, second only to rotavirus. In countries where mass vaccination against rotavirus infection is carried out, the share of NoV in the etiological structure of acute intestinal infections is increasing while the incidence of rotavirus gastroenteritis is on the decline. As a result, norovirus infection is gradually becoming the leading cause of acute gastroenteritis [1]. The virus is highly contagious; the infectious dose is 18–2800 viral particles [2]. About 700 million cases of NoV infection and more than 200 thousand deaths are registered annually in the world. To date, there is no licensed vaccine for the prevention of norovirus infection in any country in the world [3]. In 2024, the World Health Organization included norovirus infection in the list of priority infections for which a vaccine needs to be developed in the near future [4]. NoV belongs to a group of non-enveloped viruses belonging to the Caliciviridae family, Norovirus genus. The virus genome is a single-stranded RNA of positive polarity, which encodes non-structural proteins (ORF1), the major structural protein VP1 (ORF2) and the minor capsid protein VP2 (ORF3). The viral capsid has icosahedral symmetry and consists of 180 copies of the VP1 protein, which are complemented by one or two copies of the minor VP2 protein [5].
Ten NoV genetic groups and several dozen genotypes have been identified, differing in the amino acid sequences of the VP1 protein. Representatives of 5 genogroups (GI, GII, GIV, GVIII and GIX) are pathogenic for humans. The most common and clinically significant is NoV genotype GII.4, responsible for 70–80% of norovirus outbreaks worldwide in the last three decades [6].
NoV VP1 is capable of forming virus-like particles (VLPs) that are morphologically indistinguishable from the natural virus but do not contain nucleic acid. This allows such VLPs to be used as safe vaccine components. VP1 consists of two domains – S and P. The S-domain (shell) is responsible for the self-assembly of the capsid and is located inside the viral particle. The P-domain (protruding) is located on the surface, additionally stabilizes the virion structure and is represented by two subdomains – P1 and P2, which carry antigenic sites and are responsible for the binding of the virus to the structures of the host organism (human blood group antigens HBGA). Along with full-length VP1, its constituent domains are also capable of forming VLPs of different sizes [7–9].
Several vaccine options are being developed for the prevention of human norovirus infection. Most of them are based on the use of VP1 and VLPs formed by this protein or its P-domain, which enhance the immune response to VP1 [10]. However, there are many gaps in the knowledge of natural immunity to human NoV, which complicates the development of vaccines. The ability to induce immunity to a wide range of frequently circulating genotypes and protect against future emerging strains determines the effectiveness of the vaccine. At the same time, the possibility of cross-immunity to different genetic groups or genotypes within one NoV genetic group remains poorly understood [11].
Previously, we obtained recombinant NoV VP1 genotype GII.4, circulating in the territory of the Central part of Russia. Its capability of forming VLPs, as well as its properties were briefly described [12].
The aim of this study was to characterize the immunogenic properties of recombinant NoV VP1 and assess the cross-reactivity of antibodies against it with different NoV genetic variants.
Materials and methods
Expression, purification and renaturation of recombinant NoV VP1 genotype GII.4 were carried out according to the methods described earlier [12]. For immunization, 8-week-old female BALB/c mice weighing 16–18 g were used. The animals were kept in vivarium conditions in accordance with the interstate standards GOST 33216-2014 and GOST 33215-2014. Biomaterial for the study was taken from mice in compliance with the principles of humanity set out in the directives of the European Community (86/609/EC). The studies were carried out in accordance with the bioethical and ethical principles established by the Declaration of Helsinki (adopted in June 1964 and revised in October 2013). The animals were divided into 3 groups of 10 individuals: the 1st group received 0.5 ml of saline intraperitoneally; Group 2 – 10 μg VP1 in 0.5 ml of saline; Group 3 – 10 μg VP1 in 0.5 ml of saline with the addition of 100 μg of aluminum hydroxide. Animals were immunized twice with an interval of 2 weeks. Blood was collected and serum was obtained 3 weeks after the second immunization. Determination of antibodies to NoV VP1 in the blood serum of immunized mice was performed using solid-phase enzyme immunoassay. VP1 was adsorbed into the wells of the plates at a concentration of 1 μg/mL for 18 hours at a temperature of 4 °C. The plates were washed three times with phosphate-buffered saline containing 0.1% Tween-20 (PBS-T). 1 μL of blood serum was diluted with PBS-T containing 10% clarified Escherichia coli Rosetta 2 (DE3) cell lysate in 2-step solutions and added to the wells of the plates in a volume of 100 μL, followed by incubation for 1 h at 37 °C. Then the wells of the plates were washed with PBS-T and 100 μL of a solution of horseradish root peroxidase-conjugated rabbit antibodies against the total fraction of mouse immunoglobulins (IMTEK, Russia) or against immunoglobulins class M (IgM) (Elabscience, China) were added, incubated for 1 h at 37 °C and washed with PBS-T. The reaction was visualized by adding 100 μL of a solution of 0.04% tetramethylbenzidine containing 0.002 hydrogen peroxide at pH 5.0. The plate was left in a dark place for 10 min. 50 μL of 1N H2SO4 were added to each well and the optical density (OD) was measured in dual-wavelength mode, at a main wavelength of 450 nm and at a reference wavelength of 620 nm in an Infinite M200 Pro microplate reader (TECAN, Austria) with Magellan 7.2 software (TECAN, Austria). Blood serum from mice that received 0.5 mL of saline intraperitoneally was used as a negative control. All tests were performed three times each. An OD value exceeding the average value of the negative control multiplied by 3 was considered a positive reaction.
To determine the antibody avidity, blood serum samples were tested in duplicate by the enzyme immunoassay with the modifications described below. Before adding the conjugate, 100 μL of 8 M urea in PBS-T were added to one of the two wells of each sample, incubated for 3 min, washed 5 times, and analyzed as described above. The avidity index was calculated as the ratio of the OD value in the wells with 8 M urea to the OD value in the wells without urea obtained for one blood serum, expressed as a percentage.
To perform immunoelectron microscopy, guinea pig antibodies to NoV VP1 were obtained. Guinea pigs were immunized subcutaneously twice with an interval of 2 weeks. 500 μg of recombinant NoV VP1 mixed with 5 mg of aluminum hydroxide were administered. Twenty-one days after the booster immunization, blood was collected and the total immunoglobulin fraction was isolated by salting out with 33% ammonium sulfate, followed by dialysis of the precipitate against distilled water; 50 μl of the isolated antibodies were adsorbed for 60 min on copper grids for electron microscopy (EM) coated with parlodium padding. The grids were washed with distilled water and placed on drops of coprofiltrates containing NoV of the genotypes GII.4, GII.7, GII.17, GI.3 or echovirus 30 as a negative control. The mixture was incubated for 60 min at 37 °C, stained with an aqueous solution of 2% uranyl acetate (pH 4.5) and analyzed for the presence of viral particles using an HT7700 microscope (Hitachi, Japan). The study used the coprofiltrates stored in the research collection of the I.N. Blokhina Nizhny Novgorod Research Institute of Epidemiology and Microbiology with the presence of viruses confirmed by electron microscopy and polymerase chain reaction with reverse transcription.
Statistical data processing was performed using Prism software (GraphPad Software) and Microsoft Excel. Differences were considered statistically significant at p ≤ 0.05.
Results
All immunized animals had antibodies against the NoV VP1 protein in different titers in their blood serum. In mice injected with physiological saline, antibodies against VP1 were not detected. IgM antibody titers on the 21st day after double immunization of mice with recombinant VP1 ranged from 1 : 256 to 1 : 1024. The arithmetic mean titer was 1 : 474 (Fig. 1). In mice immunized with VP1 mixed with aluminum hydroxide, the antibody titer was 2 times higher, but the differences were not statistically significant. Even higher antibody titers were obtained when analyzing their total fraction. Fig. 2 shows that the average titer of total antibodies was 1 : 1869. In certain samples, the titer values reached 1 : 8000. Immunization of animals with recombinant protein with the addition of an adjuvant further increased the antibody titers. In this case, they fluctuated in animals from 1 : 512 to 1 : 32,768. The arithmetic mean titer was 1 : 13,158. The geometric mean titers characterizing the intensity of population immunity were 1 : 1024 and 1 : 9410, respectively.
Fig. 1. IgM antibody titers in mice immunized with recombinant VP1 protein in the absence (a) and presence (b) of aluminium hydroxide.
* – statistically significant differences (p < 0.05).
Рис. 1. Титры IgM-антител у мышей, иммунизированных рекомбинантным VP1 в отсутствие (а) и в присутствии (б) гидроокиси алюминия.
* – статистически значимые различия (p < 0,05).
Fig. 2. Titers of total antibodies in mice immunized with recombinant VP1 protein in the absence (a) and presence (b) of aluminium hydroxide.
* – statistically significant differences (p < 0.05).
Рис. 2. Титры суммарных антител у мышей, иммунизированных рекомбинантным VP1 в отсутствие (а) и в присутствии (б) гидроокиси алюминия.
* – статистически значимые различия (p < 0,05).
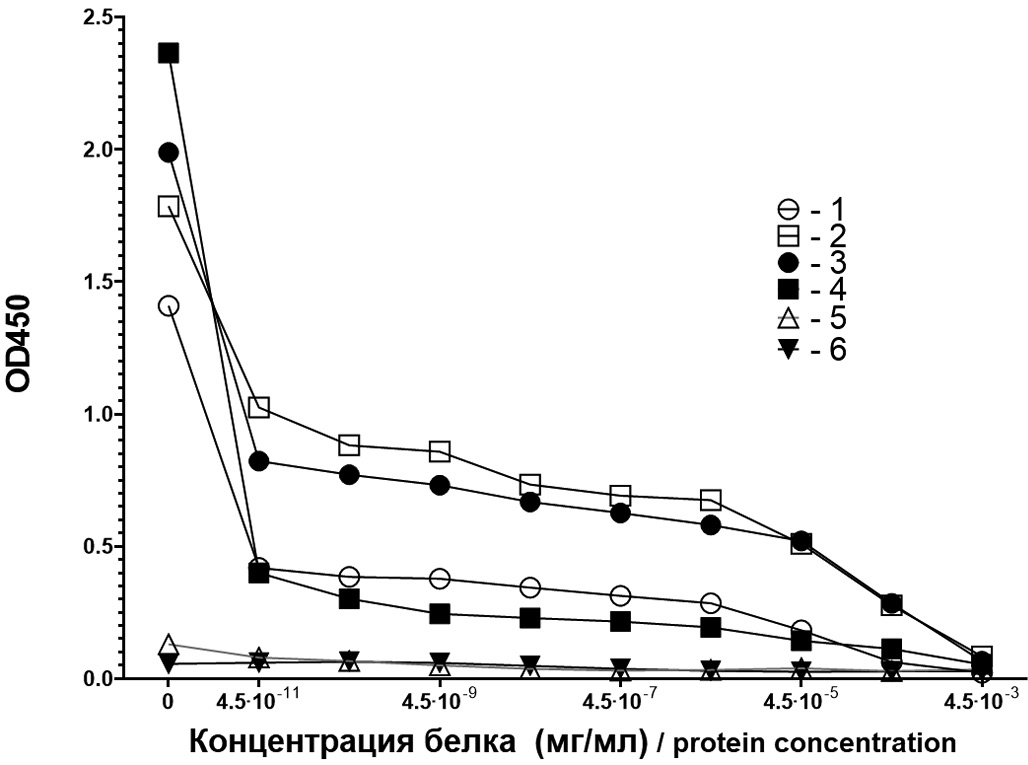
The specificity of both total antibodies against recombinant VP1 and IgM antibodies was assessed. In a competitive reaction, when antibodies against VP1 were preincubated with recombinant VP1 in increasing concentrations, it was shown that mouse antibodies were neutralized by the recombinant protein by 95% at a protein concentration exceeding 4.5 × 10−4 mg/ml (Fig. 3). The neutralization curves of total antibodies and IgM antibodies produced by mice after immunization in the absence and presence of the used adjuvant are shown in Fig. 3.
Fig. 3. Neutralization of mouse total antibodies (AB) and IgM AB by recombinant VP1 protein.
1 – total AB after immunization without adjuvant; 2 – same with adjuvant; 3 – IgM AB after immunization without adjuvant; 4 – same with adjuvant; 5 – total AB of unimmunized mice; 6 – IgM-AB of unimmunized mice.
Рис. 3. Нейтрализация рекомбинантным VP1 мышиных суммарных антител (АТ) и АТ класса IgM.
1 – суммарные АТ после иммунизации без адъюванта; 2 – то же с адъювантом; 3 – IgM-АТ после иммунизации без адъюванта; 4 – то же с адъювантом; 5 – суммарные АТ неиммунизированных мышей; 6 – IgM-АТ неиммунизированных мышей.
When determining the avidity of antibodies against VP1, it was shown that total antibodies against VP1 obtained after immunization of mice without adjuvant had an avidity index of 55.22%. The use of adjuvant led to an increase in the avidity index of antibodies to 83.99%. An avidity index that is near 100% indicates a high probability of the production of virus-neutralizing antibodies by animals. IgM antibodies against VP1 had a much lower avidity index, amounting to 9.08% in mice immunized with VP1 with adjuvant. The avidity index of IgM antibodies after immunization with recombinant VP1 without adjuvant was 8.11%.
The possibility of cross-reactions of serum antibodies against VP1 with NoV of different genotypes was studied using solid-phase immunoelectron microscopy. Viruses isolated from the feces of patients with norovirus gastroenteritis and enterovirus meningitis collected in the territory of the Russian Federation were used. EM grids were sensitized with antibodies against NoV VP1 genotype GII.4 and tested for interaction with NoV of other genotypes. EM showed the presence of viral particles with a diameter of 40–50 nm when applying coprofiltrates containing NoV of genotypes GII.4, GII.7, GII.17, but not GI.3, to grids coated with immunoglobulins (Fig. 5). A coprofiltrate containing Echovirus 30 was used as a control for binding specificity; no viral particles were detected in it using grids coated with immunoglobulins against NoV VP1. The obtained results indicate the binding of serum polyclonal IgG against VP1 genotype GII.4 with NoV genotypes GII.7, GII.17, but not with NoV genotype GI.3, i.e. NoV genogroup GI.
Fig. 4. Comparison of avidity indices of total antibodies (1 – immunization with VP1 alone, 2 – VP1 together with adjuvant) and IgM class antibodies (3 – immunization with VP1 alone, 4 – VP1 together with adjuvant).
Рис. 4. Сравнение индексов авидности суммарных АТ (1 – иммунизация только VP1, 2 – VP1 совместно с адъювантом) и АТ класса IgM (3 – иммунизация только VP1, 4 – VP1 совместно с адъювантом).
Fig. 5. Electron micrographs of norovirus particles interacting with antibodies against norovirus VP1 protein.
a – norovirus (NoV) genotype GII.4; b – NoV genotype GII.17. c – NoV genotype GII.7; d – GI.3; e – Echovirus 30.
Рис. 5. Электронные микрофотографии норовирусных частиц, взаимодействующих с антителами против VP1 норовируса.
а – норовирус (НВ) генотипа GII.4; б – НВ генотипа GII.17; в – HB генотипа GII.7; г – GI.3; д – Echovirus 30.
Discussion
The NoV VP1 protein contains several antigenic determinants (epitopes) that determine its immunogenic properties. The S-domain is formed by the N-terminal residues from amino acids 1 to 225. Residues from 50 to 225 are folded into an 8-chain antiparallel β-structure, characteristic of the capsid proteins of many viruses. The polypeptide chain starting from the 225th amino acid forms the P1 and P2 subdomains. Using monoclonal antibodies, a fairly conservative region constructed from 60 amino acids and containing B-cell antigenic determinants was found in the N-terminal part of the S-domain of NoV VP1 genogroup II [13, 14]. Several cross-reactive antibodies targeting the conserved region of the P-domain have also been described and characterized [15–20]. Among them, antibodies that react with both linear and conformational epitopes were found [16]. It was shown that neutralizing antibodies can be formed into some of the identified epitopes, which is important for the implementation of the antiviral B-cell response [21, 22]. Five antigenic regions constructed from variable B-cell epitopes and localized in the structure of the P2 subdomain on its outer surface were identified [23]. This region of the P2 subdomain is characterized by a high mutation rate, which is probably the result of selective pressure from the host immunity and contains epitopes that induce the formation of neutralizing antibodies [24].
In our studies using in silico analysis, several possible T-cell epitopes presented by HLA class I and II molecules were identified in the NoV VP1 genotype GII.4 Sydney [P16], circulating in Russia and reproduced by us as a recombinant protein, i.e. capable of activating both the cytotoxic T-cell response and the T-helper response. Modeling of the structure and its analysis also allowed us to detect 2 linear and 47 conformational B-cell epitopes of VP1 in the absence of its allergenicity [25].
The presence of the indicated epitopes ensures the formation of a B-cell response, which was registered in our study. Total antibodies, produced in high titers in response to immunization with recombinant VP1, had high avidity, which most likely suggests their virus-neutralizing properties. The use of an adjuvant brought the avidity index closer to 100%. It should be noted that high immunogenicity is also due to the ability of VP1 to form VLPs, which we demonstrated earlier [12]. The expected lower titers and avidity of IgM antibodies, compared to total antibodies, formed during immunization of animals with recombinant VP1. This is probably due to the relatively short period of immunization of animals and, as a result, the insufficient maturity of the developed immune response.
To study the cross-reactions of antibodies against NoV VP1, surrogate models based on the interaction of antibodies with VLPs formed by VP1 of different NoV genotypes are mainly used. Recent studies have shown that monoclonal IgM antibodies were able to bind VLPs belonging to the GI (GI.1, GI.2, GI.3) and GII (GII.3, GII.4, GII.6, GII.13 or GII.17) genetic groups. Cross-reactivity of monoclonal IgA antibodies was recorded only between genotypes within the GII genetic group. Monoclonal IgG antibodies had cross-reactivity with VLPs obtained from VP1 of different GII genotypes and one genotype GI.3 [26, 27]. We used an original approach to study the cross-reactivity of antibodies against VP1 GII.4 based on the use of immunoelectron microscopy isolated from patients with norovirus gastroenteritis. The results obtained confirm the cross-reactivity of IgG antibodies against VP1 GII.4 with other genotypes. However, their neutralizing activity remains unclear. Earlier, when studying the immune response to immunization with NoV VLPs, it was shown that both cross-reactive but non-neutralizing antibodies and more narrowly reactive neutralizing antibodies are present in human blood serum [28].
Conclusion
Thus, the obtained recombinant VP1, which forms VLPs and induces a strong immune response with the formation of highly avid polyclonal cross-reacting antibodies, can be considered as an antigen component of the prototype of a candidate vaccine against human NoV of the most common genetic group GII.
About the authors
Vladislav A. Lapin
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Author for correspondence.
Email: fridens.95@yandex.ru
ORCID iD: 0000-0002-5905-5722
Junior Researcher, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodDmitry V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: novikov.dv75@mail.ru
ORCID iD: 0000-0001-7049-6935
PhD, Leading Researcher, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodAlexander Yu. Kashnikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: mevirfc@mail.ru
ORCID iD: 0000-0003-1033-7347
Researcher, laboratory of molecular epidemiology of viral infections
Russian Federation, 603950, Nizhny NovgorodNatalia V. Epifanova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: epifanovanv@mail.ru
ORCID iD: 0000-0001-7679-8029
PhD, Leading Researcher, laboratory of molecular epidemiology of viral infections
Russian Federation, 603950, Nizhny NovgorodNadezhda A. Novikova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: novikova_na@mail.ru
ORCID iD: 0000-0002-3710-6648
Professor. Head of the laboratory of molecular epidemiology of viral infections
Russian Federation, 603950, Nizhny NovgorodEkaterina V. Mokhonova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: ekaterinamohonova@yandex.ru
ORCID iD: 0000-0002-9742-7646
Researcher, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodDmitry A. Melentev
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: dim-melente@yandex.ru
ORCID iD: 0000-0002-2441-6874
Junior Researcher, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodMaria I. Tsyganova
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: maria_che@mail.ru
ORCID iD: 0000-0002-2811-6844
PhD, Leading Researcher, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodDmitry E. Zaitsev
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: mitya.zaitseff@yandex.ru
ORCID iD: 0000-0002-7663-6924
Senior Lab Assistant, laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodViktor V. Novikov
Academician I.N. Blokhina Nizhny Novgorod Scientific Research Institute of Epidemiology and Microbiology of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare (Rospotrebnadzor)
Email: mbre@mail.ru
ORCID iD: 0000-0002-2449-7213
Professor. Head of the laboratory of immunochemistry
Russian Federation, 603950, Nizhny NovgorodReferences
- Sergevnin V.I. Modern trends in long-term dynamics of the acute intestinal infections incidence of bacterial and viral etiology. Epidemiologiya i vaktsinoprofilaktika. 2020; 19(4): 14–9. https://doi.org/10.31631/2073-3046-2020-19-4-14-19 https://elibrary.ru/zejihk (in Russian)
- Hall A.J., Wikswo M.E., Pringle K. Vital signs: foodborne norovirus outbreaks – United States, 2009–2012. MMWR Morb. Mortal Wkly Rep. 2014; 63(22): 491–5.
- Netzler N.E., Enosi Tuipulotu D., White P.A. Norovirus antivirals: Where are we now? Med. Res. Rev. 2019; 39(3): 860–86. https://doi.org/10.1002/med.21545
- Hasso-Agopsowicz M., Hwang A., Hollm-Delgado M.G., Umbelino-Walker I., Karron R.A., Rao R., et al. Identifying WHO global priority endemic pathogens for vaccine research and development using multi-criteria decision analysis. EBioMedicine. 2024; 110: 105424. https://doi.org/10.1016/j.ebiom.2024.105424
- Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999; 286(5438): 287–90. https://doi.org/10.1126/science.286.5438.287
- Chhabra P., de Graaf M., Parra G.I., Chan M.C., Green K., Martella V., et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019; 100(10): 1393–406. https://doi.org/10.1099/jgv.0.001318
- Tan M., Jiang X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005; 79(22): 14017–30. https://doi.org/10.1128/JVI.79.22.14017-14030.2005
- Bertolotti-Ciarlet A., White L.J., Chen R., Prasad B.V., Estes M.K. Structural requirements for the assembly of Norwalk virus-like particles. J. Virol. 2002; 76(8): 4044–55. https://doi.org/10.1128/JVI.76.8.4044-4055.2002
- Fang H., Tan M., Xia M., Wang L., Jiang X. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One. 2013; 8(5): e63269. https://doi.org/10.1371/journal.pone.0063269
- Mohsen M.O., Gomes A.C., Vogel M., Bachmann M.F. Interaction of viral capsid-derived virus-like particles with the innate immune system. Vaccines (Basel). 2018; 6(3): 37. https://doi.org/10.3390/vaccines6030037
- Cates J.E., Vinjé J., Parashar U., Hall A.J. Recent advances in human norovirus research and implications for candidate vaccines. Expert. Rev. Vaccines. 2020; 19(6): 539–48. https://doi.org/10.1080/14760584.2020.1777860
- Lapin V.A., Novikov D.V., Mokhonova E.V., Melentyev D.A., Tsyganova M.I., Zaitsev D.E., et al. Production of recombinant norovirus VP1 protein and its antigenic and immunogenic properties. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2024; 101(5): 661–7. 2024; 101(5): 661–7. https://doi.org/10.36233/0372-9311-552 https://elibrary.ru/ubmktf (in Russian)
- Parra G.I., Azure J., Fischer R., Bok K., Sandoval-Jaime C., Sosnovtsev S.V., et al. Identification of a broadly cross-reactive epitope in the inner shell of the norovirus capsid. PLoS One. 2013; 8(6): e67592. https://doi.org/10.1371/journal.pone.0067592
- Li X., Zhou R., Tian X., Li H., Zhou Z. Characterization of a cross-reactive monoclonal antibody against Norovirus genogroups I, II, III and V. Virus Res. 2010; 151(2): 142–7. https://doi.org/10.1016/j.virusres.2010.04.005
- Parra G.I., Abente E.J., Sandoval-Jaime C., Sosnovtsev S.V., Bok K., Green K.Y. Multiple antigenic sites are involved in blocking the interaction of GII.4 Norovirus capsid with ABH histo-blood group antigens. J. Virol. 2012; 86(13): 7414–26. https://doi.org/10.1128/JVI.06729-11
- Parker T.D., Kitamoto N., Tanaka T., Hutson A.M., Estes M.K. Identification of Genogroup I and Genogroup II broadly reactive epitopes on the norovirus capsid. J. Virol. 2005; 79(11): 7402–9. https://doi.org/10.1128/JVI.79.11.7402-7409.2005
- Shiota T., Okame M., Takanashi S., Khamrin P., Takagi M., Satou K., et al. Characterization of a broadly reactive monoclonal antibody against norovirus genogroups I and II: recognition of a novel conformational epitope. J. Virol. 2007; 81(21): 12298–306. https://doi.org/10.1128/JVI.01196-07
- Li X., Zhou R., Wang Y., Sheng H., Tian X., Li H., et al. Identification and characterization of a native epitope common to norovirus strains GII/4, GII/7 and GII/8. Virus Res. 2009; 140(1-2): 188–93. https://doi.org/10.1016/j.virusres.2009.01.015
- Yoda T., Terano Y., Suzuki Y., Yamazaki K., Oishi I., Utagawa E., et al. Characterization of monoclonal antibodies generated against Norwalk virus GII capsid protein expressed in Escherichia coli. Microbiol. Immunol. 2000; 44(11): 905–14. https://doi.org/10.1111/j.1348-0421.2000.tb02579.x
- Almanza H., Cubillos C., Angulo I., Mateos F., Castón J.R., van der Poel W.H., et al. Self-assembly of the recombinant capsid protein of a swine norovirus into virus-like particles and evaluation of monoclonal antibodies cross-reactive with a human strain from genogroup II. J. Clin. Microbiol. 2008; 46(12): 3971–9. https://doi.org/10.1128/jcm.01204-08
- Lindesmith L.C., McDaniel J.R., Changela A., Verardi R., Kerr S.A., Costantini V., et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity. 2019; 50(6): 1530–41. https://doi.org/10.1016/j.immuni.2019.05.007
- Hansman G.S., Taylor D.W., McLellan J.S., Smith T.J., Georgiev I., Tame J.R., et al. Structural basis for broad detection of genogroup II noroviruses by a monoclonal antibody that binds to a site occluded in the viral particle. J. Virol. 2012; 86(7): 3635–46. https://doi.org/10.1128/JVI.06868-11
- Ford-Siltz L.A., Tohma K., Parra G.I. Understanding the relationship between norovirus diversity and immunity. Gut Microbes. 2021; 13(1): 1–13. https://doi.org/10.1080/19490976.2021.1900994
- Winder N., Gohar S., Muthana M. Norovirus: An overview of virology and preventative measures. Viruses. 2022; 14(12): 2811. https://doi.org/10.3390/v14122811
- Zharova A.M.D., Talayev V.Yu., Perenkov A.D., Zaichenko I.Ye., Svetlova M.V., Babaykina O.N., et al. In silico analysis of the antigenic properties of norovirus GII.4 Sydney [P16] VP1 protein. Opera Med. Physiol. 2023; 10(3): 140–51. https://doi.org/10.24412/2500-2295-2023-3-140-151
- Alvarado G., Salmen W., Ettayebi K., Hu L., Sankaran B., Estes M.K., et al. Broadly cross-reactive human antibodies that inhibit genogroup I and II noroviruses. Nat. Commun. 2021; 12(1): 4320. https://doi.org/10.1038/s41467-021-24649-w
- Park J., Lindesmith L.C., Olia A.S., Costantini V.P., Brewer-Jensen P.D., Mallory M.L., et al. Broadly neutralizing antibodies targeting pandemic GII.4 variants or seven GII genotypes of human norovirus. Sci. Transl. Med. 2025; 17(788): eads8214. https://doi.org/10.1126/scitranslmed.ads8214
- Lindesmith L.C., McDaniel J.R., Changela A., Verardi R., Kerr S.A., Costantini V., et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity. 2019; 50(6): 1530–41.e8. https://doi.org/10.1016/j.immuni.2019.05.007
Supplementary files