Identification of human genes potentially involved in the pathogenesis of viral hepatitis C based on multi-network bioinformatics analysis
- Authors: Anufrieva E.V.1, Ostankova Y.V.1, Davydenko V.S.1, Schemelev A.N.1, Totolian A.A.1
-
Affiliations:
- St. Petersburg Pasteur Institute
- Issue: Vol 70, No 3 (2025)
- Pages: 267-281
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16747
- DOI: https://doi.org/10.36233/0507-4088-314
- EDN: https://elibrary.ru/KFJUSV
- ID: 16747
Cite item
Abstract
Aim. The aim of this study was to search for human genes potentially involved in the pathogenesis of hepatitis C by multi-network bioinformatics linkage analysis of proteins involved in the stages of hepatitis C virus (HСV) attachment and entry.
Materials and methods. A number of web applications with complementary algorithms and databases were used to analyze genetic and protein-protein networks. The following genes were used as basic genes: CD81, CLDN1, LDLR, OCLN, SCARB1, the products of which are involved in interaction with viral glycoproteins E1 and E2 at the stage of HCV attachment and penetration into the cell. Data analysis was performed, including a two-stage scoring ranking of the identified candidate genes based on their interaction with basic genes and their presence in the results of network analysis of different web resources.
Results. Candidate genes were initially identified using three web resources: HumanNet – 100 candidate genes, GeneMania – 20, STRING – 98. Based on the intersection of the three web resources, the total number of candidate genes associated with basic genes was 170. The total number of genes with a rank higher than 4 points was 35. Candidate genes were grouped into functional sets: cellular barriers and intercellular contacts (17 genes, 48.6%); lipid metabolism and lipoproteins (9 genes, 25.7%); immune response and interaction with the virus (5 genes, 14.3%); signaling pathways, proteolysis and cytoskeleton (4 genes, 11.4%). The following candidate genes potentially involved in the pathogenesis of HCV have been identified: APOA1, CLDN3, APOE, LIPC, LRPAP1, CSNK1E, APOB, CD19, CLDN6, CLDN9, ESAM, F11R, IFITM1, LDLRAP1, PCSK9, TJP1, CD9, CLDN11, CLDN17, CLDN2, CLDN5, IGSF8, MMP2, PDZK1, ADAM10, APOA2, C3, CLDN12, DAB1, GJB1, ITGB1, MYLIP, NEDD4L, PTGFRN.
Conclusion. In the future, a detailed study of the functional features and polymorphic variants of the identified genes using bioinformatics and laboratory methods can significantly expand current understanding of the involvement of human genes in the development of HCV infection and discover new targets for the development of drugs and therapeutic strategies.
Full Text
Introduction
Approximately 50 million people worldwide are infected with chronic viral hepatitis C, and nearly 400,000 people die annually from this virus, mainly due to liver cirrhosis and hepatocellular carcinoma (HCC1 [1].
Genetic heterogeneity characteristic of the hepatitis C virus (HCV) is the main reason for the rapid adaptation of the viral population in the host organism to various selection factors. As a result of the aforementioned, spontaneous elimination of the virus occurs with complete restoration of the body’s homeostasis in 20% of infection cases, while in 80% of patients, the pathological process progresses into a chronic disease, and in 30% of patients the chronic course of the disease is complicated by liver cirrhosis or HCC in 5% [2]. However, it is impossible to explain this solely by the genetic diversity of the virus, as cases are not uncommon where the development of the disease differed among infected individuals, despite being infected with the same virus isolates, for example, during outbreaks and/or accidents, as well as through the transfusion of contaminated blood. The heterogeneity of clinical manifestations is apparently due to the complex interaction of both HCV-specific factors and individual characteristics of the host organism. Genetically determined mechanisms play a decisive role in ensuring phenotypic variability in response to viral infection. Mutations and polymorphic variants affecting penetration HCV and/or disease progression have been described for several host genes [3, 4]. A significant problem in research aimed at studying the interaction between the pathogen and the host is the high level of individual genetic variability of the host organism, which includes polymorphic gene variants and a range of mutational events potentially associated with the pathogenesis of HCV. Conducting a comprehensive experimental analysis of the human genome to identify correlations between host genetic markers and the characteristics of the infectious process presents a methodological challenge, the resolution of which is extremely difficult within the existing capabilities of laboratory diagnostics. The solution to this problem may be the preliminary identification of genes potentially involved in the pathogenesis of HCV using in silico methods. At the same time, the systematic nature of the approach should be determined by linking bioinformatics research to the viral life cycle.
The entry of HCV into the cell largely depends on the interaction of host lipoprotein components and the viral envelope glycoproteins E1 and E2 with receptors expressed on the surface of hepatocytes. Over the past two decades, researchers have identified numerous human molecules involved in the processes leading from the attachment of the virus to the hepatocyte to receptor-mediated endocytosis of the viral particle and endosomal fusion [5, 6]. The virus attaches to surface proteoglycans, lipid receptors LDLR/VLDLR, saprophytic receptor SR-BI (scavenger receptor class B member 1, product of the SCARB1 gene), and CD81.
Following lateral translocation of virions to the tight junction zone, the tight junction proteins claudin-1 (CLDN1) and occludin (OCLN) are required for entry. Clathrin-mediated endocytosis engulfs HCV particles, which fuse with endosomal membranes following a decrease in pH [5]. Thus, the entry of HCV into the cell is a multi-step process that requires at least the four aforementioned entry factors: the saprophytic receptor SR-BI, the tetraspanin CD81, and the CLDN1 and OCLN proteins [7]. As an alternative to the SR-BI receptor, the virus can also use the low-density lipoprotein receptor (LDLR) for attachment [8].
Based on the above mentioned, genes encoding proteins known to be associated with virus entry into the cell may subsequently be considered as a basis for searching candidate genes for the pathogenesis of HCV using bioinformatics analysis methods.
The aim of the study is to search for human genes potentially involved in the pathogenesis of HCV infection using a multi-network bioinformatics analysis of the interactions of proteins involved in the stages of virus attachment and entry into the cell.
Materials and methods
The basis for this study was a group of genes, the products of which, according to the literature, are involved in the interaction with viral glycoproteins E1 and E2 at the stage of HCV entry into the cell: CD81, CLDN1, LDLR, OCLN, SCARB1. The specified genes were used as the baseline for the analysis.
The present study used a range of web resources to evaluate genetic and protein-protein interactions, the algorithms and databases of which provide a complementary approach [9]:
- HumanNetv3 (https://www.inetbio.org/humannet/pcs.php) ‒ A web platform designed for the search and analysis of candidate genes associated with diseases. This tool is focused on studying the human genome and proteome, making it particularly useful for research related to human diseases.
- STRING (https://string-db.org) – An algorithm designed for searching and analyzing gene and protein interactions, is based on the integration of data from various sources.
- GeneMANIA (https://genemania.org) ‒ A web resource that allows not only the use of built-in databases but also the addition of custom interaction networks, making the analysis more flexible and specific.
Within the framework of this study, evaluation parameters for all the aforementioned programs were established as described earlier [9].
Point ranking system
For an objective analysis of the functional significance of candidate genes, a ranking assessment system was developed, based on score ranking, analogous to the ranking system proposed earlier [9, 10]. This method consists of two stages.
At the first stage, a standardized assessment of the association between candidate genes was conducted based on uniform parameters within each web resource used. Candidate genes were assigned one point for each identified interaction with any of the three basic genes: CD81, CLDN1 or OCLN. Since SCARB1 and LDLR are interchangeable during HCV entry into the cell, 1 point was also assigned for interaction with either of them. Thus, the highest number of points that can be assigned to a candidate gene is 4, provided that this gene has pathways with all four main HCV entry molecules (CD81, CLDN1, OCLN, SCARB1/LDLR).
The second stage involved the evaluation of the frequency of detection of candidate genes in interaction networks constructed by independent web resources, which allowed determining the degree of their functional significance using different algorithms. If the candidate gene was detected using all three web resources, it was awarded 3 points; if it was detected in only two web resources, it was awarded 2 points. Points were not awarded for presence in only one web resource.
According to the following formula “HumanNet_Scores + STRING_Scores + GeneMANIA_Scores + Web_Resource_Intersection_Scores”, the minimum passing score is 4. Thus, for each analyzed candidate gene, minimum criteria for the significance of interactions with basic genes were established, confirmed by data from at least two independent web resources.
Results
Analysis with HumanNetv3
The total number of potential candidate genes based on protein-protein interactions was 648 genes. Since HumanNetv3 considers the first hundred candidates for subsequent functional analysis, genes with an AUROC prediction up to a false positive rate of 1% were selected for further work (Fig. 1). At the same time, false positive candidates were excluded, and the selected genes were ranked based on the assessment of their relationships with the basic genes CD81, CLDN1, LDLR, OCLN, SCARB1 genes involved in the pathogenesis of HCV. The threshold value (relationship score) used to identify the most likely candidate genes, reflecting proximity to other genes according to the weighted neighbor rule, was 2.6471. The selected genes were ranked by the threshold value from 2.6471 to 8.4774.
Fig. 1. AUROC prediction of the candidate genes involved in the pathogenesis of HCV infection to the level of false positive results of 1%.
Рис. 1. AUROC-прогноз генов-кандидатов патогенеза гепатита С до уровня ложноположительных результатов в 1%.
The network of protein-protein interactions of potential candidate genes is presented in Fig. 2.
Fig. 2. Potential candidate genes involved in the infection and/or development of the HCV infection (HumanNetv3, threshold score > 2.6471).
Рис. 2. Потенциальные гены-кандидаты, участвующие в инфицировании и/или развитии гепатита С (HumanNetv3, пороговая оценка > 2,6471).
Most of the identified candidate genes are involved in lipid metabolism, immune response, maintenance of intercellular contacts, and are also associated with cell signaling and antiviral defense.
Analysis with STRING
The STRING algorithm identifies candidate genes by analyzing protein-protein interactions, so the further characterization of these genes and basic genes within this resource covers the physical interactions and pathways of their protein products.
When analyzing the interactions of candidate genes with basic genes, the reliability level of the pathways varied from 0.400 to 0.998.
As a result of the analysis, 103 candidate genes were identified, with a total of 1343 pathways between the genes. The average number of pathways per gene was 24.9, and the clustering coefficient reached 0.908. It is important to note that the number of detected pathways significantly exceeds the expected value for this set of genes, which would be 142 (Fig. 3).
Fig. 3. Protein-protein interaction network based on the web resource STRING.
Connections built based on databases are indicated in blue, while those based on experimental data are shown in pink.
Рис. 3. Сеть белок-белковых взаимодействий на основании веб-ресурса STRING.
Голубым цветом выделены связи, построенные на основании баз данных, розовым – на основании экспериментальных данных.
The majority of the candidate genes belong to the families of apolipoproteins, tight junction proteins and immune regulators, with additional contributions to endocytosis and signal transduction. During the analysis of genetic and protein-protein networks, the pathways between the identified genes were cut. As a result, 98 genes were found to be potentially significant according to STRING.
Analysis with GeneMANIA
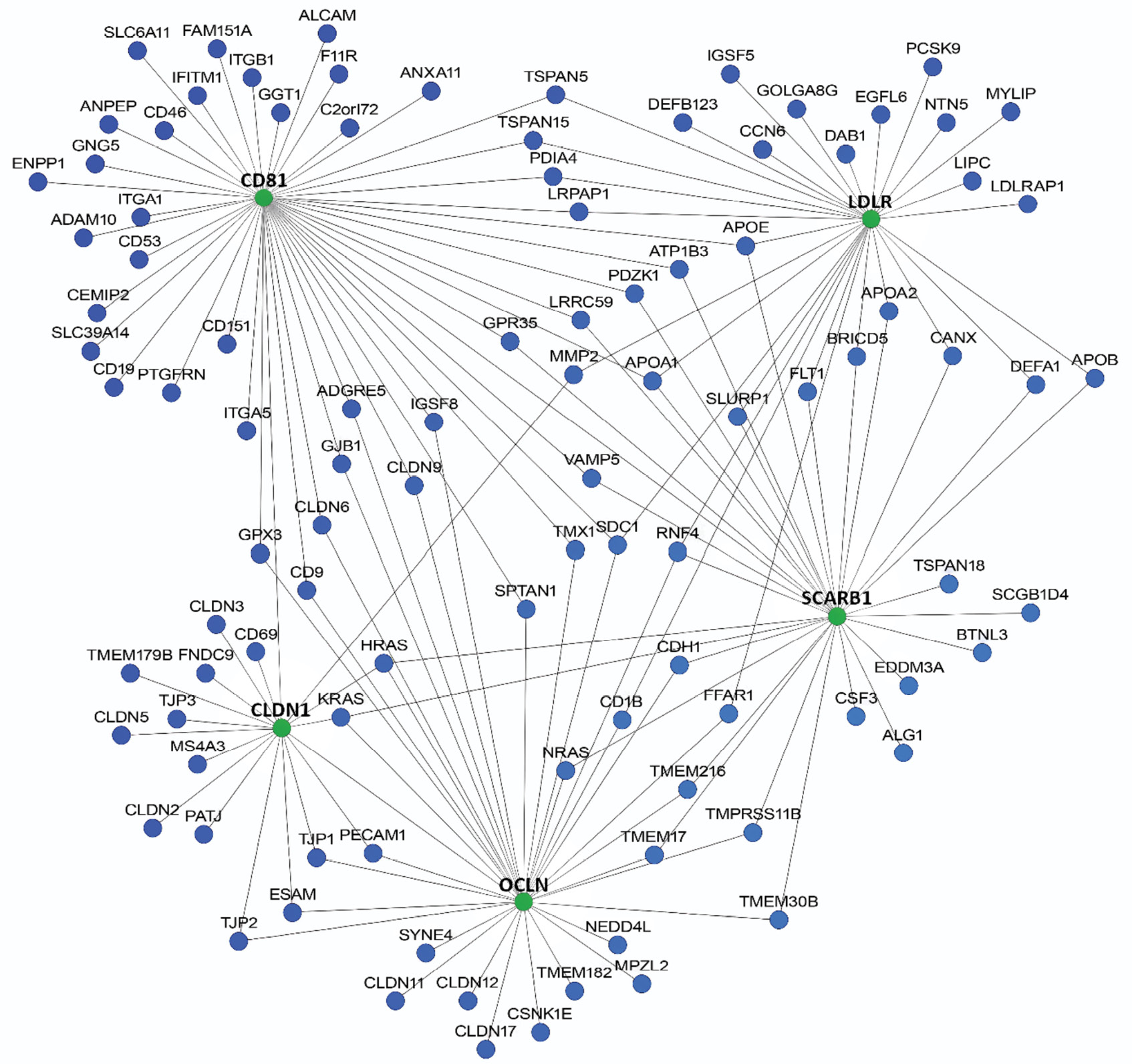
As a result of the analysis, 100 potential candidate genes interacting with HCV entry factors into the cell and potentially involved in the pathogenesis of HCV were identified. The level of confidence in the pathways ranged from 0.00038 to 1. The wide range of levels is due to the fact that this web resource includes not only human genes in the analysis but also their homologs from different species. Fig. 4 shows the protein-protein interactions of the products of candidate genes and basic genes.
Fig. 4. Comprehensive protein-protein interaction network based on the web resource GeneMania.
Basic genes are marked with shading.
The size of the nodes reflects the number of connections of the specified protein/gene based on all interactions. Physical associations are indicated in red, genetic associations are indicated in green, co-expression is indicated in purple, participation in biological pathways is indicated in blue, predicted interactions are indicated in orange.
Рис. 4. Комплексная сеть белок-белковых взаимодействий на основании веб-ресурса GeneMania.
Базовые гены помечены штриховкой. Размер шариков отражает количество связей указанного белка/гена на основании всех взаимодействий. Красным цветом отмечены физические связи, зеленым – генетические связи, фиолетовым – коэкспрессия, голубым – участие в биологических путях, оранжевым – предсказанные взаимодействия.
When evaluating the structure of the complex protein-protein interaction network, it was found that the majority of pathways (77.64%) are based on physical interactions between the products of candidate genes and basic genes. In second place in terms of significance are pathways driven by co-expression (8.01%). The remaining types of interactions include pathways: predicted by algorithms (5.37%), genetic interactions (2.87%), and participation in common biological pathways (1.88%). From further analysis, candidate genes whose pathways with other genes are based solely on co-localization and shared protein domains were excluded, as these criteria do not provide sufficient information to assess the functional significance of the interactions. Such pathways accounted for 4.23% of the total.
Most of the presented candidate genes are associated with lipid transport, immune response, and intercellular adhesion. During the analysis of genetic and protein-protein networks, redundant pathways and those between the identified genes were removed. As a result, 20 genes were found to be potentially significant according to GeneMANIA.
Point ranking system
According to the obtained evaluation results using three web resources, candidate genes were identified: HumanNet – 100 candidate genes, STRING – 98, GeneMANIA – 20. Comprehensive data analysis allowed the identification of 170 candidate genes that are associated with basic genes. The Table presents the most significant candidate genes that reached the threshold value (total score ≥ 4) based on the results of the comprehensive interaction assessment.
Table. Rank count for connection contributions in each web resource for candidate genes potentially involved in the pathogenesis of HCV infection
Таблица. Ранговый подсчет вклада связей в каждом веб-ресурсе для генов-кандидатов, потенциально участвующих в патогенезе ВГС-инфекции
Candidate genes Гены-кандидаты | Score (Баллы) | Total Итого | |||
HumanNet | STRING | GeneMANIA | Common in web resources Пересечение в веб-ресурсах | ||
TJP2 | 2 | 1 | 4 | 3 | 10 |
APOA1 | 2 | 1 | 3 | 3 | 9 |
CLDN3 | 1 | 2 | 3 | 3 | 9 |
APOE | 2 | 1 | 2 | 3 | 8 |
LIPC | 1 | 1 | 3 | 3 | 8 |
LRPAP1 | 2 | 1 | 2 | 3 | 8 |
CSNK1E | 1 | 1 | 2 | 3 | 7 |
APOB | 1 | 1 | 1 | 3 | 6 |
CD19 | 1 | 1 | 1 | 3 | 6 |
CLDN6 | 2 | 2 | 0 | 2 | 6 |
CLDN9 | 2 | 2 | 0 | 2 | 6 |
ESAM | 2 | 2 | 0 | 2 | 6 |
F11R | 1 | 0 | 3 | 2 | 6 |
IFITM1 | 1 | 1 | 1 | 3 | 6 |
LDLRAP1 | 1 | 1 | 1 | 3 | 6 |
PCSK9 | 1 | 1 | 1 | 3 | 6 |
TJP1 | 2 | 2 | 0 | 2 | 6 |
CD9 | 2 | 1 | 0 | 2 | 5 |
CLDN11 | 1 | 2 | 0 | 2 | 5 |
CLDN17 | 1 | 2 | 0 | 2 | 5 |
CLDN2 | 1 | 2 | 0 | 2 | 5 |
CLDN5 | 1 | 2 | 0 | 2 | 5 |
IGSF8 | 2 | 1 | 0 | 2 | 5 |
MMP2 | 2 | 1 | 0 | 2 | 5 |
PDZK1 | 2 | 1 | 0 | 2 | 5 |
ADAM10 | 1 | 1 | 0 | 2 | 4 |
APOA2 | 1 | 1 | 0 | 2 | 4 |
C3 | 0 | 1 | 1 | 2 | 4 |
CLDN12 | 1 | 1 | 0 | 2 | 4 |
DAB1 | 1 | 1 | 0 | 2 | 4 |
GJB1 | 1 | 1 | 0 | 2 | 4 |
ITGB1 | 1 | 1 | 0 | 2 | 4 |
MYLIP | 1 | 1 | 0 | 2 | 4 |
NEDD4L | 1 | 1 | 0 | 2 | 4 |
PTGFRN | 1 | 1 | 0 | 2 | 4 |
The total number of candidate genes with a rank of 4 and higher was 35 genes. The identified candidate genes were grouped into functional sets: cellular barriers and intercellular contacts (17 genes, 48.6%); lipid metabolism and lipoproteins (9 genes, 25.7%); immune response and virus interaction (5 genes, 14.3%); signaling pathways, proteolysis and cytoskeleton (4 genes, 11.4%).
Discussion
As part of this study, a comprehensive bioinformatics analysis was conducted to identify candidate genes potentially associated with HCV entry into the host cell through interactions with the CD81, CLDN1, OCLN, SCARB1/LDLR genes. In addition to the main factors of HCV entry into the cell, numerous cofactors involved in virus attachment and subsequent fusion with the host cell have been described. As a rule, these are genes that are expressed in almost all tissues and organs [11].
The candidate genes identified in this study can be divided into four groups regarding their possible role in HCV infection, taking into account their known biological functions and involvement in processes related to viral infection, immune response, cellular barriers and metabolism:
1) genes facilitating HCV entry into the cell;
2) genes involved in the assembly of HCV virions;
3) genes involved in the HCV immune response and chronicity;
4) genes involved in the regulation of signaling pathways.
1. Genes facilitating HCV entry into the cell
The first group of genes, which includes proteins of cell barriers and intercellular junctions, plays a key role in maintaining the structure and function of epithelial and endothelial barriers, which are important for the entry and spread of HCV in the body. The following genes were included in the group (with corresponding ranking scores in parentheses): TJP2 (10), CLDN3 (9), TJP1 (6), CLDN6 (6), CLDN9 (6), F11R (6), ESAM (6), CLDN2 (5), CLDN5 (5), CLDN11 (5), CLDN17 (5), MMP2 (5), IGSF8 (5), PDZK1 (5), CLDN12 (4), GJB1 (4), DAB1 (4).
Genes of tight junctions encode proteins that form specialized intercellular pathways, which regulate intercellular transport and maintain cell polarity [12]. During HCV infection, these proteins play a key role, as the virus uses them to enter hepatocytes. Claudins (CLDN) are a family of transmembrane proteins that form tight junctions, which control the transport of ions and molecules between cells. Members of the claudin family are highly conserved, especially in the first extracellular loop (EL1), which is important for interaction with HCV [13].
It is known that CLDN6 and CLDN9 are expressed in the liver, the main organ of HCV replication, as well as in peripheral blood mononuclear cells, an additional site of viral replication. Claudins CLDN6 and CLDN9 are capable of penetration of the virus into cells, especially in the absence or suppression of the main co-receptor CLDN1, unlike CLDN2, CLDN3, CLDN4, CLDN7, CLDN11, CLDN12, CLDN15, CLDN17 and CLDN23 [14]. However, claudins, like the key components of tight junctions (TJ), play an important role in the emergence and development of HCC. Disruption of claudin expression regulation leads to loss of intercellular adhesion and aberrant cell signaling, which are closely associated with cancer cell invasion, migration, and epithelial-mesenchymal transition. CLDN1, CLDN3, CLDN4, CLDN5, CLDN6, CLDN7, CLDN9, CLDN10, CLDN11, CLDN14 and CLDN17 proteins are abnormally expressed in HCC, which stimulates disease progression [15], and indirectly indicates involvement in the pathogenesis of HCV. The TJP1 and TJP2 genes (zonula occludens proteins) encode proteins that link tight junction components to the cytoskeleton, ensuring the structural stability of the barrier and participating in the regulation of cell polarity. These proteins are involved in the earliest stages of HCV entry into the cell [13, 16].
The PDZK1 gene encodes an adapter protein that regulates the localization and function of tight junction proteins, including the regulation of transporters and receptors in the liver. SR-BI is the main receptor for high-density lipoproteins in the liver, where its expression is primarily regulated at the post-transcriptional level through interaction with the PDZK1 scaffolding protein. Stable suppression of PDZK1 expression using microRNAs in human hepatoma cells significantly reduces their susceptibility to HCV infection. The results of the conducted study indicate the indirect involvement of PDZK1 in the entry of HCV into the cell, through interaction with the SR-BI receptor and modulation of its functional activity as a viral co-receptor [17].
ESAM and F11R (JAM-A) are adhesion molecules that regulate intercellular contacts and the migration of immune cells, which is important for the inflammatory response during infection. It has been found that the ESAM gene is one of the regulators of the differentiation of hepatic stellate cells into myofibroblasts [18]. Thus, in chronic liver inflammation caused by HCV, changes in ESAM expression may contribute to the development of fibrosis and impaired vascular function. The F11R protein is localized in tight junctions between endothelial and epithelial cells, where it regulates intercellular adhesion and barrier function, affecting vascular permeability and the migration of immune cells into tissues, including the liver [19]. It has been shown that F11R plays a key role in regulating leukocyte infiltration during liver inflammation and fibrogenesis [20], indicating the important role of F11R in maintaining vascular integrity and controlling inflammation in chronic liver damage, including HCV infection.
The MMP2 gene encodes matrix metalloproteinase (MMP) 2. In chronic hepatitis C, pathological accumulation of extracellular matrix is a hallmark of liver fibrosis. MMPs play a crucial role in the remodeling of the extracellular matrix, which allows considering MMP2 as a potential marker of liver inflammation. A significant increase in MMP2 levels was observed in patients with chronic hepatitis C compared to the control group [21]. Another study showed that excessive synthesis of MMP2 contributes to liver inflammation and fibrosis in HCV infection [22].
The DAB1 gene encodes the reelin signaling adapter, which is involved in cell migration and development, particularly in neuronal differentiation. Reelin is a secreted extracellular glycoprotein that plays an important role in brain development. In several studies, the expression of reelin in human liver stellate cells has been described [23]. The expression of reelin significantly correlates with the stages of liver fibrosis and may potentially contribute to fibrogenesis in patients infected with HCV [24].
Data on the direct role of the IGSF8 gene in HCV infection are limited; however, IGSF8 is known as an immunoglobulin-like protein involved in cell adhesion and immune communication. This protein interacts with tetraspanins CD81 and CD9 and may regulate their role in certain cellular functions, including cell migration, which could also be relevant for viral infections [25].
Connexin (Cx32), encoded by the GJB1 gene, forms channels for intercellular communication and plays an important role in regulating the growth and differentiation of liver cells, as well as in controlling liver cancer stem cells. It has been shown that Cx32 regulates the expansion of the liver stem cell population and affects the progression of HCC through the PI3K/Akt signaling pathway [26].
HCV infects hepatocytes, which form tight cell contacts regulated by the proteins described above. Disruption of tight junction structure or alteration of CLDN and TJP expression may facilitate viral entry into cells and the spread of infection. Moreover, changes in adhesive molecules and remodeling enzymes (such as MMP2) affect liver inflammation and fibrosis characteristic of HCV. Thus, this group of genes provides the structural and functional basis of cellular barriers that HCV uses and modifies for successful replication and persistence in the host.
2. Genes involved in the assembly of HCV virions
The group included genes (with corresponding ranking scores in parentheses) encoding enzymes, receptors, and regulators of lipid metabolism, as well as apolipoproteins: APOA1 (9), APOE (8), LIPC (8), LRPAP1 (8), APOB (6), PCSK9 (6), LDLRAP1 (6), APOA2 (4), MYLIP (4).
The second functional group includes genes related to lipid metabolism and lipoprotein metabolism. These genes play an important role in the formation of HCV lipoviral particles, as well as in the regulation of lipid metabolism, which significantly affects the virus’s life cycle and the pathogenesis of the infection. HCV particles are secreted as lipoviral particles. These hybrid particles are enriched with triglycerides and cholesterol esters and consist of structural proteins and human apolipoproteins, including ApoB (product of the APOB gene), ApoE (product of the APOE gene), ApoA-I (product of the APOA1 gene), ApoA-II (product of the APOA2 gene), and ApoC-I [27]. The ApoE protein apparently has a dual function for HCV. Firstly, as an integral part of HCV particles, ApoE facilitates the entry of the virus into hepatocytes by mediating high-affinity interactions with cell surface molecules such as LDLR and SR-BI receptors [28]. Secondly, ApoE is necessary for the production of infectious HCV particles [29].
Another candidate gene, PCSK9, which encodes the subtilisin/kexin type 9 protease, demonstrates an antiviral effect on HCV in cells and suppresses the expression level of mouse hepatic CD81 in vivo. Therefore, it is suggested that PCSK9 activity may modulate HCV infectivity in humans [30]. PCSK9 also regulates the amount of LDLR on the cell surface, which affects lipid metabolism [30], and may potentially be related to the pathogenesis of HCV.
The LIPC gene (liver lipase), which encodes an enzyme that hydrolyzes triglycerides and phospholipids in lipoproteins, affects lipid metabolism and, indirectly, viral infection. It has been shown that lipase affects the course of the HCV life cycle by reducing intracellular triglyceride levels, which are essential for the virus life cycle [32].
Despite the absence of direct evidence for the involvement of the LDLRAP1, LRPAP1 and MYLIP genes in the process of HCV entry into the cell, their functional pathway with the regulation of LDLR receptor activity suggests a potential indirect influence on the mechanisms of viral infection.
The LDLRAP1 adaptor protein (LDL receptor adaptor protein 1) mediates the internalization of LDL receptors [33], which may affect the intracellular transport of viral particles. The MYLIP protein, on the other hand, regulates the degradation of LDL receptors, thereby affecting cholesterol levels and possibly the viral life cycle [34]. Since LDLR is considered one of the main factors for HCV entry, it can be assumed that changes in the expression or activity of LDLRAP1 and MYLIP may modulate the availability of LDLR on the cell surface, thereby indirectly affecting the efficiency of viral infection. The LRPAP1 gene encodes a protein that interacts with lipoprotein receptors and is involved in their proper folding and localization. In the context of viral infections, including HCV, recent studies suggest that LRPAP1 may play a role in suppressing the innate immune response. Secreted LRPAP1 binds to the IFNAR1 receptor and promotes its degradation, which weakens the interferon-induced antiviral response. This promotes the enhancement of infections by various viruses, both RNA and DNA-containing, including the hepatitis B virus [35].
3. Genes involved in the HCV immune response and chronicity
This group included genes (with corresponding ranking scores in parentheses) associated with the immune response and interaction with HCV: CD19 (6), IFITM1 (6), CD9 (5), C3 (4), PTGFRN (4).
These genes play a key role in the formation of antiviral immunity and the mechanisms of HCV persistence in the human body, participating in virus recognition, immune cell activation, opsonization and suppression of viral replication. Their functional characteristics largely determine the outcome of the infection—spontaneous recovery or chronicity of the process [36].
The IFITM1 gene, encoding an interferon (IFN)-inducible protein that restricts viral entry into cells, including HCV, by altering membrane structure, significantly limits HCV entry into hepatocytes by disrupting sequential interactions between the virus and key host co-receptors, particularly CD81 and OCLN. Moreover, IFITM1 exerts antiviral effects against a range of viruses, including HCV, in the early stages of infection [37].
Protein C3 (beta-1-C-globulin) is a component of the complement system, involved in the opsonization of viral particles and the activation of the inflammatory response, playing a key role in both the classical and alternative pathways of complement activation. This system is an important part of both innate and adaptive immunity, ensuring the elimination of pathogens [38, 39]. Numerous studies confirm the involvement of the complement system in the pathogenesis of a wide range of liver pathologies, including viral hepatitis, hepatocyte damage and regeneration processes, fibrogenesis, alcohol-associated liver injury and ischemic/reperfusion injuries [38]. Particular interest is shown by recent studies demonstrating a statistically significant decrease in complement C3 concentration in patients with chronic hepatitis C, cirrhosis, and extrahepatic manifestations [39].
Tetraspanin D19 is a marker of B cells, playing a key role in the activation and differentiation of B lymphocytes, which may be important for the production of antibodies against HCV. The CD9 gene encodes a tetraspanin involved in the formation of membrane microdomains and influencing cell adhesion, migration, and may participate in the process of viral entry and transmission. Currently, there are no direct studies showing a link between the CD9 and CD19 genes and the course of HCV infection. However, it is known that HCV is capable of infecting B lymphocytes that express CD19, as well as other blood cells. Infection with HCV can cause B-cell lymphoproliferative diseases, indicating a possible interaction between the virus and B-cells expressing CD19. In chronic hepatitis C, the predominant association of HCV with B cells is mediated by the complement system, primarily through complement receptor 2 (CD21), in conjunction with the CD19 and CD81 complex [40]. The CD81 protein binds with other tetraspanins, particularly with CD9. This complex influences the induction of IFN-α (a member of the type I interferon family) by HCV-infected cells [41].
Currently, the PTGFRN gene, which encodes a protein that regulates the prostaglandin F2α receptor, is poorly described. It is suggested that it is associated with metastasis in some types of cancer [42]. However, its role in the pathogenesis of HCV has not yet been described.
4. Genes involved in the regulation of signaling pathways
The last group of genes (with corresponding ranking scores in parentheses) is associated with the regulation of signaling pathways, proteolysis, and cytoskeletal processes, which play an important role in cell adhesion, migration, intracellular signaling, and potentially in interaction with HCV: CSNK1E (7), ADAM10 (4), ITGB1 (4), NEDD4L (4).
The ITGB1 protein (Integrin beta-1) is a component of integrin receptors, ensuring cell adhesion to the extracellular matrix and participating in signaling pathways that affect cell migration and survival, as well as interaction with the virus. ITGB1 interacts with CD81 and acts as a cofactor for entry during HCV entry. This protein also participates in the formation of a functional complex to regulate membrane organization and receptor mobility [5].
The ADAM10 gene encodes a metalloproteinase whose main function is proteolysis of various cell proteins, including receptors and adhesion molecules, which affects cell migration and may facilitate viral entry. ADAM10 associates with CD81, SR-BI, and acts as an entry cofactor [43].
The NEDD4L gene encodes an E3 ubiquitin ligase (NEDD4) that regulates the degradation of proteins involved in cellular signaling and homeostasis, which can affect the viral life cycle and immune response. Despite the lack of data confirming the involvement of NEDD4 in the pathogenesis of HCV, it is known to be significant in liver fibrosis in female mice [44] and is well described as a necessary protein for the replication of certain RNA viruses. For example, NEDD4 interacts with proline-rich motifs in the late budding domains of the Ebola virus, rabies virus and HIV [45]. Moreover, NEDD4 positively regulates the antiviral innate immunity, promotes the production of interferons type I and pro-inflammatory cytokines, some of which are known to be involved in the development of liver inflammation [46].
The CSNK1E gene (Casein kinase 1 epsilon) encodes a serine/threonine protein kinase involved in the regulation of cell cycles, circadian rhythms, and signaling pathways that may affect viral replication and the cellular response to infection. It is known that CSNK1E is involved in the regulation of circadian rhythms through the phosphorylation of PER and CRY proteins [47]. Disruption of circadian rhythms can affect hepatocyte metabolism, creating a favorable environment for HCV replication. CSNK1E also affects lipid metabolism and the development of liver steatosis [48], which is often associated with HCV, accelerating fibrogenesis [49]. Thus, although there is no data indicating the influence of serine/threonine protein kinase on the course of HCV infection, it may be involved in the pathogenesis of the disease and the immune response through the aforementioned mechanisms.
Thus, the genes of the fourth group regulate key cellular processes that HCV uses for successful entry, replication and evasion of the immune response. For example, ADAM10 may facilitate the cleavage of receptors necessary for viral entry, while ITGB1 is involved in the organization of the cytoskeleton and migration, which is important for the spread of the infection. CSNK1E and NEDD4L influence intracellular signaling pathways and proteolysis, regulating the stability and activity of proteins involved in antiviral defense and the cell cycle.
A comprehensive bioinformatics analysis allowed for the identification of candidate genes potentially involved in the infection and progression of HCV. Despite the fact that in silico methods played a key role in the preliminary selection, the primary objective of this study was to identify genes critically important for the biological processes under investigation. Particular interest lies in candidate genes whose contribution to the pathogenesis of HCV has not been previously described. Presumably, the products of these genes may participate in the infectious process indirectly, which opens new avenues for studying the mechanisms of infection. The obtained results highlight the importance of including the identified genes in studies of the pathogenesis of HCV.
It is necessary for the next stage of the research to conduct a comprehensive bioinformatics analysis of the 35 identified genes, including the assessment of expression, localization of the identified gene products at the cellular level, as well as the characterization of their functional interactions and biological pathways. Such an approach will allow for the identification of key molecular determinants that modulate the infectious process and pathogenesis. The analysis of the significance of polymorphic variants of the identified candidate genes for pathogenesis also holds undeniable scientific interest. In the future, such studies will contribute to the identification of new genetic factors affecting susceptibility to HCV, the discovery of new markers of disease progression, and the expansion of our understanding of the fundamental mechanisms of HCV infection. The final stage should be the experimental validation of the functional role of the identified candidate genes in the pathogenesis of HCV.
Conclusion
In the present study, comprehensive bioinformatics analysis and subsequent ranking helped identify 35 candidate genes associated with proteins involved in the stages of virus attachment and entry into the cell, and potentially participating in the pathogenesis of the infection. The role of most of the identified genes as co-receptors for HCV is already known, but particular interest lies in the genes whose products have not previously been considered as associated with HCV infection. The obtained results indicate their potential contribution to the processes of viral entry and HCV chronicity. A detailed study of the functional characteristics and polymorphic variants of the identified genes using bioinformatics and experimental methods can significantly expand current understanding the genetic mechanisms of interaction between the virus and the host organism and open new prospects for the development of therapeutic strategies.
1 World Health Organization. Hepatitis C. Key facts. Hepatitis C (who.int). Avaliable at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
About the authors
Ekaterina V. Anufrieva
St. Petersburg Pasteur Institute
Author for correspondence.
Email: kate.an21@yandex.ru
ORCID iD: 0009-0002-1882-529X
Junior Researcher, Laboratory of Immunology and Virology HIV Infection, Postgraduate Student
Russian Federation, St PetersburgYulia V. Ostankova
St. Petersburg Pasteur Institute
Email: shenna1@yandex.ru
ORCID iD: 0000-0003-2270-8897
PhD, Senior Researcher at the Laboratory of Molecular Immunology, Head of the Laboratory of Immunology and Virology HIV Infection
Russian Federation, St PetersburgVladimir S. Davydenko
St. Petersburg Pasteur Institute
Email: vladimir_david@mail.ru
ORCID iD: 0000-0003-0078-9681
Junior Researcher, Laboratory of Immunology and Virology HIV Infection, Postgraduate Student
Russian Federation, St PetersburgAlexandr N. Schemelev
St. Petersburg Pasteur Institute
Email: tvildorm@gmail.com
ORCID iD: 0000-0002-3139-3674
PhD, Junior Researcher, Laboratory of Immunology and Virology HIV Infection, Postgraduate Student
Russian Federation, St PetersburgAreg A. Totolian
St. Petersburg Pasteur Institute
Email: totolian@pasteurorg.ru
ORCID iD: 0000-0003-4571-8799
Academician of the Russian Academy of Sciences, PhD, MD (Medicine), Professor, Head at the Laboratory of Molecular Immunology, Director, head Department of Immunology, First St. Petersburg State Medical University named after Academician I.P. Pavlov
Russian Federation, St PetersburgReferences
- Valutite D., Ostankova Y., Semenov A., Lyalina L., Totolian A. Distribution of primary resistance mutations in Saint Petersburg in patients with chronic hepatitis C. Diagnostics (Basel). 2022; 12(5): 1054. https://doi.org/10.3390/diagnostics12051054
- Guss D., Sherigar J., Rosen P., Mohanty S.R. Diagnosis and management of hepatitis C Infection in primary care settings. J. Gen. Intern. Med. 2018; 33(4): 551–7. https://doi.org/10.1007/s11606-017-4280-y
- Stanislovaitiene D., Lesauskaite V., Zaliuniene D., Smalinskiene A., Gustiene O., Zaliaduonyte-Peksiene D., et al. SCARB1 single nucleotide polymorphism (rs5888) is associated with serum lipid profile and myocardial infarction in an age- and gender-dependent manner. Lipids Health Dis. 2013; 12: 24. https://doi.org/10.1186/1476-511X-12-24
- Itakura J., Nagayama K., Enomoto N., Sakamoto N., Tazawa J., Izumi N., et al. CD81 nucleotide mutation in hepatocellular carcinoma and lack of CD81 polymorphism in patients at stages of hepatitis C virus infection. J. Med. Virol. 2001; 63(1): 22–8.
- Gerold G., Moeller R., Pietschmann T. Hepatitis C virus entry: protein interactions and fusion determinants governing productive hepatocyte invasion. Cold Spring Harb. Perspect. Med. 2020; 10(2): a036830. https://doi.org/10.1101/cshperspect.a036830
- Zeisel M.B., Felmlee D.J., Baumert T.F. Hepatitis C virus entry. Curr. Top. Microbiol. Immunol. 2013; 369: 87–112. https://doi.org/10.1007/978-3-642-27340-7_4
- Carriquí-Madroñal B., Sheldon J., Duven M., Stegmann C., Cirksena K., Wyler E., et al. The matrix metalloproteinase ADAM10 supports hepatitis C virus entry and cell-to-cell spread via its sheddase activity. PLoS Pathog. 2023; 19(11): e1011759. https://doi.org/10.1371/journal.ppat.1011759
- Yamamoto S., Fukuhara T., Ono C., Uemura K., Kawachi Y., Shiokawa M., et al. Lipoprotein receptors redundantly participate in entry of hepatitis C virus. PLoS Pathog. 2016; 12(5): e1005610. https://doi.org/10.1371/journal.ppat.1005610
- Davydenko V.S., Ostankova Yu.V., Shchemelev A.N., Anufrieva E.V., Kushnareva V.V., Totolian A.A. Identification of human genes interacting with HIV attachment receptors and potentially involved in disease pathogenesis based on multi-network bioinformatics analysis. VICh-infektsiya i immunosupressii. 2024; 16(4): 28–44. https://doi.org/10.22328/2077-9828-2024-16-4-28-44 (in Russian)
- Davydenko V.S., Ostankova Y.V., Schemelev A.N., Anufrieva E.V., Kushnareva V.V., Totolian A.A. Bioinformatically analyzed relationships between specific human genes associated with HIV attachment. Russian Journal of Infection and Immunity. 2024; 14(6): 1153–68. https://doi.org/10.15789/2220-7619-BAR-17830
- Colpitts C.C., Tsai P.L., Zeisel M.B. Hepatitis C virus entry: an intriguingly complex and highly regulated process. Int. J. Mol. Sci. 2020; 21(6): 2091. https://doi.org/10.3390/ijms21062091
- Gumbiner B.M. Breaking through the tight junction barrier. J. Cell. Biol. 1993; 123(6 Pt. 2): 1631–3. https://doi.org/10.1083/jcb.123.6.1631
- Mailly L., Baumert T.F. Hepatitis C virus infection and tight junction proteins: The ties that bind. Biochim. Biophys. Acta Biomembr. 2020; 1862(7): 183296. https://doi.org/10.1016/j.bbamem.2020.183296
- Zheng A., Yuan F., Li Y., Zhu F., Hou P., Li J., et al. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 2007; 81(22): 12465–71. https://doi.org/10.1128/JVI.01457-07
- Wang W., Zhou Y., Li W., Quan C., Li Y. Claudins and hepatocellular carcinoma. Biomed. Pharmacother. 2024; 171: 116109. https://doi.org/10.1016/j.biopha.2023.116109
- Park J.H., Park S., Yang J.S., Kwon O.S., Kim S., Jang S.K. Discovery of cellular proteins required for the early steps of HCV infection using integrative genomics. PLoS One. 2013; 8(4): e60333. https://doi.org/10.1371/journal.pone.0060333
- Eyre N.S., Drummer H.E., Beard M.R. The SR-BI partner PDZK1 facilitates hepatitis C virus entry. PLoS Pathog. 2010; 6(10): e1001130. https://doi.org/10.1371/journal.ppat.1001130
- Li X., Wang Q., Ai L., Cheng K. Unraveling the activation process and core driver genes of HSCs during cirrhosis by single-cell transcriptome. Exp. Biol. Med. (Maywood). 2023; 248(16): 1414–24. https://doi.org/10.1177/15353702231191109
- Rosager A.M., Sørensen M.D., Dahlrot R.H., Boldt H.B., Hansen S., Lathia J.D., et al. Expression and prognostic value of JAM-A in gliomas. J. Neurooncol. 2017; 135(1): 107–17. https://doi.org/10.1007/s11060-017-2555-0
- Brozat J.F., Brandt E.F., Stark M., Fischer P., Wirtz T.H., Flaßhove A., et al. JAM-A is a multifaceted regulator in hepatic fibrogenesis, supporting LSEC integrity and stellate cell quiescence. Liver Int. 2022; 42(5): 1185–203. https://doi.org/10.1111/liv.15187
- Abdel-Latif M.S. Plasma levels of matrix metalloproteinase (MMP)-2, MMP-9 and tumor necrosis factor-α in chronic hepatitis C virus patients. Open Microbiol. J. 2015; 9: 136–40. https://doi.org/10.2174/1874285801509010136
- Neuman M.G., Schmilovitz-Weiss H., Hilzenrat N., Bourliere M., Marcellin P., Trepo C., et al. Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int. J. Hepatol. 2012; 2012: 231210. https://doi.org/10.1155/2012/231210
- Samama B., Boehm N. Reelin immunoreactivity in lymphatics and liver during development and adult life. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005; 285(1): 595–9. https://doi.org/10.1002/ar.a.20202
- Carotti S., Perrone G., Amato M., Vespasiani Gentilucci U., Righi D., Francesconi M., et al. Reelin expression in human liver of patients with chronic hepatitis C infection. Eur. J. Histochem. 2017; 61(1): 2745. https://doi.org/10.4081/ejh.2017.2745
- Fan Y., Pionneau C., Cocozza F., Boëlle P.Y., Chardonnet S., Charrin S., et al. Differential proteomics argues against a general role for CD9, CD81 or CD63 in the sorting of proteins into extracellular vesicles. J. Extracell. Vesicles. 2023; 12(8): e12352. https://doi.org/10.1002/jev2.12352
- Li H., Wang B., Qi B., Jiang G., Qin M., Yu M. Connexin32 regulates expansion of liver cancer stem cells via the PI3K/Akt signaling pathway. Oncol. Rep. 2022; 48(3): 166. https://doi.org/10.3892/or.2022.8381
- Lee J.Y., Acosta E.G., Stoeck I.K., Long G., Hiet M.S., Mueller B., et al. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J. Virol. 2014; 88(21): 12422–37. https://doi.org/10.1128/JVI.01660-14
- Zeisel M.B., Felmlee D.J., Baumert T.F. Hepatitis C virus entry. Curr. Top. Microbiol. Immunol. 2013; 369: 87–112. https://doi.org/10.1007/978-3-642-27340-7_4
- Jiang J., Luo G. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J. Virol. 2009; 83(24): 12680–91. https://doi.org/10.1128/JVI.01476-09
- Labonté P., Begley S., Guévin C., Asselin M.C., Nassoury N., Mayer G., et al. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology. 2009; 50(1): 17–24. https://doi.org/10.1002/hep.22911
- Malyarevskaya O.V., Namitokov A.M., Kruchinova S.V., Kosmacheva E.D. PCSK9 inhibitors: role in reducing cardiovascular diseases. Yuzhno-Rossiiskii zhurnal terapevticheskoi praktiki. 2022; 3(2): 32–40. https://doi.org/10.21886/2712-8156-2022-3-2-32-40 (in Russian)
- Desrochers G.F., Filip R., Bastianelli M., Stern T., Pezacki J.P. microRNA-27b regulates hepatic lipase enzyme LIPC and reduces triglyceride degradation during hepatitis C virus infection. J. Biol. Chem. 2022; 298(6): 101983. https://doi.org/10.1016/j.jbc.2022.101983
- Hubacek J.A., Hyatt T. ARH missense polymorphisms and plasma cholesterol levels. Clin. Chem. Lab. Med. 2004; 42(9): 989–90. https://doi.org/10.1515/CCLM.2004.200
- Lindholm D., Bornhauser B.C., Korhonen L. Mylip makes an Idol turn into regulation of LDL receptor. Cell. Mol. Life Sci. 2009; 66(21): 3399–402. https://doi.org/10.1007/s00018-009-0127-y
- Ostankova Y.V., Serikova E.N., Anufrieva E.V., Basina V.V., Mashkov I.A., Shirshova N.Yu., et al. Prognostic assessment of hepatocellular carcinoma development based on the determination of human IFNAR-1 gene polymorphism and/or its expression. Klinicheskaya laboratornaya diagnostika. 2024; 69(7): 349–57. https://doi.org/10.51620/0869-2084-2024-69-7-349-357 https://elibrary.ru/lcckcx (in Russian)
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J. Clin. Invest. 2009; 119(7): 1745–54. https://doi.org/10.1172/JCI39133
- Narayana S.K., Helbig K.J., McCartney E.M., Eyre N.S., Bull R.A., Eltahla A., et al. The interferon-induced transmembrane proteins, IFITM1, IFITM2, and IFITM3 inhibit hepatitis C virus entry. J. Biol. Chem. 2015; 290(43): 25946–59. https://doi.org/10.1074/jbc.M115.657346
- Mazumdar B., Kim H., Meyer K., Bose S.K., Di Bisceglie A.M., Ray R.B., et al. Hepatitis C virus proteins inhibit C3 complement production. J. Virol. 2012; 86(4): 2221–8. https://doi.org/10.1128/JVI.06577-11
- Assem N.M., Mohammed A.I., Barry H.M.A., El Sayed I.E.T., Elmadbouh I. Serum cystatin C is an early renal dysfunction biomarker in patients with hepatitis C virus. Egypt Liver J. 2022; 12(1): 67. https://doi.org/10.1186/s43066-022-00231-x
- Wang R.Y., Bare P., De Giorgi V., Matsuura K., Salam K.A., Grandinetti T., et al. Preferential association of hepatitis C virus with CD19+ B cells is mediated by complement system. Hepatology. 2016; 64(6): 1900–10. https://doi.org/10.1002/hep.28842
- Zhang S., Kodys K., Babcock G.J., Szabo G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology. 2013; 58(3): 940–9. https://doi.org/10.1002/hep.25827
- Marquez J., Dong J., Hayashi J., Serrero G. Prostaglandin F2 receptor negative regulator (PTGFRN) expression correlates with a metastatic-like phenotype in epidermoid carcinoma, pediatric medulloblastoma, and mesothelioma. J. Cell. Biochem. 2024; 125(8): e30616. https://doi.org/10.1002/jcb.30616
- Carriquí-Madroñal B., Sheldon J., Duven M., Stegmann C., Cirksena K., Wyler E., et al. The matrix metalloproteinase ADAM10 supports hepatitis C virus entry and cell-to-cell spread via its sheddase activity. PLoS Pathog. 2023; 19(11): e1011759. https://doi.org/10.1371/journal.ppat.1011759
- Chen C., Bi Y., Chen B., He S. Nedd4L signaling contributes to carbon tetrachloride-induced liver fibrosis in female mice and is associated with enteric dysbacteriosis. Gastroenterol. Rep. (Oxf.). 2025; 13: goaf022. https://doi.org/10.1093/gastro/goaf022
- Chesarino N.M., McMichael T.M., Yount J.S. E3 ubiquitin ligase NEDD4 promotes influenza virus infection by decreasing levels of the antiviral protein IFITM3. PLoS Pathog. 2015; 11(8): e1005095. https://doi.org/10.1371/journal.ppat.1005095
- Gao P., Ma X., Yuan M., Yi Y., Liu G., Wen M., et al. E3 ligase Nedd4l promotes antiviral innate immunity by catalyzing K29-linked cysteine ubiquitination of TRAF3. Nat. Commun. 2021; 12(1): 1194. https://doi.org/10.1038/s41467-021-21456-1
- Zhou L., Fitzpatrick K., Olker C., Vitaterna M.H., Turek F.W. Casein kinase 1 epsilon and circadian misalignment impact affective behaviours in mice. Eur J Neurosci. 2022; 55(9-10): 2939-2954. https://doi.org/10.1111/ejn.15456
- Leya M., Jeong H., Yang D., Ton Nu Bao T.H., Pandeya P.R., Oh S.I., et al. Hepatocyte-specific casein kinase 1 epsilon ablation ameliorates metabolic dysfunction-associated steatohepatitis by up-regulating tumor necrosis factor receptor-associated factor 3 in mice. Am. J. Pathol. 2024; 194(11): 2106–27. https://doi.org/10.1016/j.ajpath.2024.08.003
- Harrison S.A. Steatosis and chronic hepatitis C infection: mechanisms and significance. Clin. Gastroenterol. Hepatol. 2005; 3(10 Suppl. 2): S92–6. https://doi.org/10.1016/s1542-3565(05)00706-8
Supplementary files