Epidemic season 2023–2024: the palette of ARVI pathogens in some territories of the Russian Federation and WHO regions
- Authors: Burtseva E.I.1, Breslav N.V.1, Mukasheva E.A.1, Krasnoslobodtsev K.G.1, Kirillova E.S.1, Trushakova S.V.1, Komarova I.A.2, Feodoritova E.L.1, Panova A.D.1, Kisteneva L.B.1, Khlopova I.N.1, Kruzhkova I.S.1, Krepkaia A.S.1, Morozova E.O.1, Ignatieva A.V.1, Komissarov A.B.3, Tyurin I.N.4, Samkov A.A.4, Antipjat N.A.4
-
Affiliations:
- The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
- Pacific State Medical University of the Ministry of Health of the Russian Federation
- Research institute of influenza named after A.A. Smorodintsev of Ministry of Health
- Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
- Issue: Vol 70, No 3 (2025)
- Pages: 234-245
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16716
- DOI: https://doi.org/10.36233/0507-4088-302
- EDN: https://elibrary.ru/MQXOMB
- ID: 16716
Cite item
Abstract
The purpose of this work was to determine the characteristics of the circulation of acute respiratory viral infection (ARVI) pathogens during the epidemic season of 2023–2024 in the WHO regions and Russian Federation.
Materials and methods. The article uses virological, immunological, and statistical methods, analytical material from the WHO website, to assess the circulation of ARVI pathogens in the Russian Federation and WHO regions.
Results and discussion. The detection rate of positive samples in clinical materials was as follows: influenza viruses – 7.7%, ARVI – 17.1% and SARS-CoV-2 – 6.5%. According to antigenic and molecular genetic properties, the population of the dominant subtype of the influenza A(H3N2) virus was heterogeneous and differed from the vaccine strain. The favorable sensitivity profile of epidemic strains to drugs with antineuraminidase activity (oseltamivir and zanamivir) and cap-dependent endonuclease inhibitor (baloxavir marboxil) was preserved. There was a tendency to increase the activity of respiratory pathogens such as HPIV, HAdV, HRsV, HRV, HCoV and HMPV. WHO experts have developed recommendations on the composition of influenza vaccines for the countries of the Northern and the Southern hemispheres with the replacement of the component of the influenza A(H3N2) virus: A/Darwin/9/2021 with A/Thailand/8/2022 and А/Croatia/13601RV/2023 accordingly. Cases of human infection with avian and swine influenza viruses continue to be registered.
Conclusion. Against the background of a relatively low circulation of new SARS-CoV-2 variants in the 2023-2024 season, epidemic activity of influenza viruses was recorded in the countries of the Northern hemisphere at the traditional time. Globally, its onset was associated with the influenza A(H3N2) virus, followed by an increase in the activity of the influenza A(H1N1) pdm09 virus and influenza B. As in previous seasons, differences in the proportion of influenza viruses in WHO regions, including cities of the Russian Federation, were traced.
Full Text
Introduction
Against the background of the continuous circulation of the new SARS-CoV-2 coronavirus, the activity of other respiratory pathogens, including influenza, during the last 3 epidemic seasons (2020‒2023) was lower compared to the pre-pandemic period due to COVID-19 [1‒4]. Despite this, variability of SARS-CoV-2 and influenza virus populations was noted, which could not but affect the features of the epidemic process of acute respiratory viral infections (ARVI) in the post-COVID period1 [5, 6]. The study of the peculiarities of variability of viral pathogens, as well as the ability to predict evolutionary relationships can increase the level of control over the pathogens of acute respiratory viral infections and, first of all, influenza and SARS-CoV-2 viruses, for which vaccines have been developed [7]. When continuing the previously conducted annual studies, the assessment of the specific features of the development of the 2023‒2024 influenza epidemic by frequency of occurrence and spectrum of respiratory pathogens in individual territories of the Russian Federation, as well as in the regions of the World Health Organization (WHO) was of certain interest. There is an obvious necessity to analyze the results of the study of evolutionary variability of avian and swine influenza viruses, cases of which were detected in humans in different countries of the world, to assess the risks of overcoming the interspecies barrier and the possible formation of a new pandemic variant2.
The aim of the present study was to determine the peculiarities of circulation of ARVI pathogens, including influenza and SARS-CoV-2 viruses, during the epidemic season 2023‒2024 in a number of regions of the Russian Federation and the WHO.
Materials and methods
Collection of data on incidence and laboratory diagnosis of ARVI pathogens. Within the framework of epidemiological surveillance over the circulation of influenza and ARVI viruses in the Russian Center for Ecology and Epidemiology of Influenza (CEEI) of the D.I. Ivanovsky Institute of Virology of the N.F. Gamaleya NRCEM of the Russian Ministry of Health in cooperation with 10 reference bases represented by territorial departments and Centers of Hygiene and Epidemiology of Rospotrebnadzor in the European part (Novgorod Veliky, Lipetsk, Vladimir, Yaroslavl, Penza, Cheboksary), the Urals (Orenburg), Siberia (Tomsk) and the Far East (Birobidzhan and Vladivostok), we analyzed incidence and hospitalization rates in different age groups of the population, as well as the results of laboratory diagnosis of respiratory pathogens. The observation period was from week 40 (October) of 2023 to week 39 (September) of 2024.
Analysis of influenza and ARVI incidence in different age groups, isolation of influenza viruses, reverse transcription polymerase chain reaction (RT-PCR), hemagglutination inhibition reaction, assessment of sensitivity to anti-influenza drugs, and statistical processing of the results obtained have been described in previous studies [1, 2]. Within the scope of traditional surveillance, the volume of studies using laboratory methods amounted to: 44,719 clinical samples/isolates/strains for influenza viruses, 40,277 clinical samples for ARVI, and 30,890 clinical samples for SARS-CoV-2.
Whole genome amplification of influenza A and B viruses was performed according to the previously described methodology [8, 9]. The complementary DNA library was prepared using the SQK-LSK109 kit (Oxford Nanopore, UK) followed by sequencing on a MinION instrument (Oxford Nanopore, UK). Bioinformatic data processing was performed using the guppy ver.6.3.8, porechop ver.0.2.4, nanofilt ver.2.3.0, minimap2 ver.2.24, medaka ver.1.7.2 and bcftools ver.1.13 software packages.
Results
From October 2023 (week 40) to September 2024 (week 39) in the territories cooperating with CEEG, it was registered that the epidemic threshold of ARVI incidence in relation to the average indicator for the Russian Federation (72.6 per 10 thousand population) was exceeded in the periods of weeks 47‒52 of 2023, as well as weeks 2‒7, 9 and 11 of 2024. The maximum incidence rate for the total population (average value based on the data of 10 cities of the Russian Federation) was registered on week 51 of 2023 (135.9) – in the same period as in the previous season, but with a lower indicator (157.0). During this period, the frequency of positive samples for respiratory pathogens was 34.2%, including influenza viruses – 18.9%, SARS-CoV-2 – 5.7%, other ARVI – 9.6%.
The average incidence rate of acute respiratory viral infections for the whole season was slightly lower compared to that of the previous period (69.1 and 71.6, respectively); at the same time, a slight increase was noted in children 0‒2 years of age (mean 303.1 with an interval of 6.9‒553.8 and 294.4 (9.2‒475.2), respectively); in other age groups, a decrease was noted: In children 3‒6 years (264 (6.4‒409.6) and 273.9 (10.5‒425.4), respectively), school children (137.6 (5.5‒225.6) and 141.4 (9.4‒218.3), respectively) and adults (41.6 (29.0‒58.3) and 43.6 (19.4‒54.3), respectively).
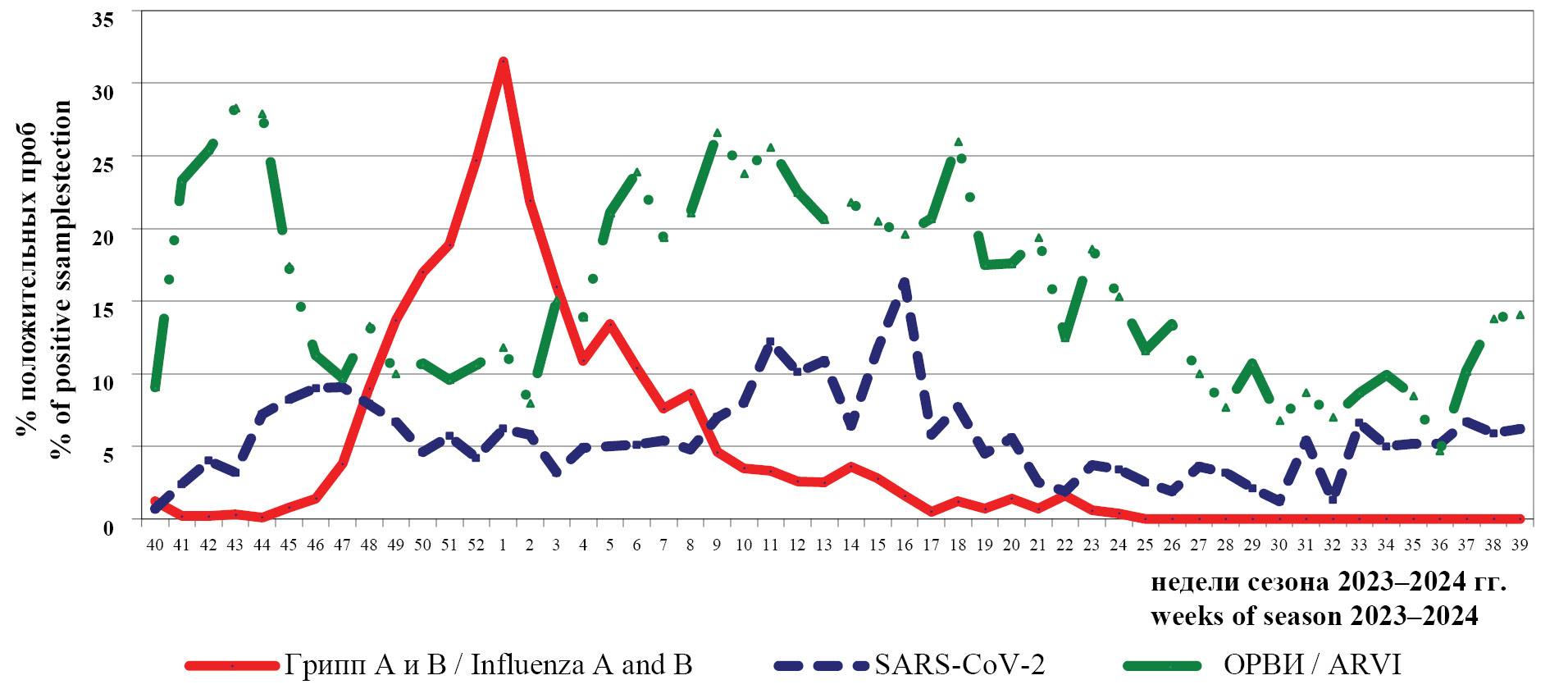
Dynamics of the frequency of positive findings for influenza A and B viruses, SARS-CoV-2 and acute respiratory viruses (including: human parainfluenza viruses types 1, 2, 3 and 4 (HPIV); human adenoviruses of groups B, C and E ((HAdV); human respiratory syncytial virus (HRSV); human rhinovirus (HRV); human bocavirus (HBoV); human metapneumovirus (HMPV); human Coronavirus (HCoV)) by RT-PCR during October 2023 – September 2024 is presented in Fig. 1.
Fig. 1. Dynamics of the frequency of detection of positive samples for virus respiratory pathogens (%) in clinical samples by RT-PCR during epidemic season 2023–2024.
Рис. 1. Динамика частоты выявления положительных образцов на вирусные респираторные возбудители (%) в клинических материалах методом ОТ-ПЦР в период эпидемического сезона 2023–2024 гг.
The epidemic season of 2023‒2024, as well as the previous one, started with high rates of positive samples for acute respiratory viruses of non-influenza etiology (28.3%). Against the background of decreasing activity of ARVI pathogens and relatively low frequency of positive samples for SARS-CoV-2 (up to 9.1%), there was an increase in the activity of influenza viruses with the maximum number of positive samples (31.5%) during the 1st week of 2024. These data indicate high epidemic activity of influenza viruses during this period and correlate with the incidence of ARVI.
During weeks 3‒24 of 2024, an increase in the activity of other respiratory pathogens was registered with the maximum rate of positive samples up to 26.0%; and up to week 27 of 2024, the rate of positive samples was not less than 10.0%. An increase in rates was also noted between week 37 and 39 of 2024 (up to 14.1%).
The highest SARS-CoV-2 activity was seen between weeks 11‒16 of 2024.
The frequency of positive findings by PCR between week 40 of 2023 and week 26 of 2024 was: 7.7% (out of 38,070 subjects) for influenza, 17.1% (out of 33,869 subjects) for ARVI, and 6.5% (out of 26,257 subjects) for SARS-CoV-2; in the period of June‒September 2024 (week 27‒39 of 2024) the rates were significantly lower and amounted to: 9.7% (out of 6408 examined) for ARVI and 4.9% for SARS-CoV-2 (out of 4633 examined). At the same time, their activity varied in different cities of the Russian Federation (Table 1).
Table 1. The results of PCR diagnostics of influenza, SARS-CoV-2 and some acute respiratory infections in the period October 2023 – September 2024 at the Centre of Ecology and Epidemiology of Influenza (CEEI) in National Research Centre of N.F. Gamaleya and in the territories of the Russian Federation cooperating with it
Таблица 1. Результаты ПЦР-диагностики гриппа, SARS-CoV-2 и некоторых ОРВИ в период октября 2023 г. – сентября 2024 г. в ЦЭЭГ НИЦЭМ им. Н.Ф. Гамалеи и на сотрудничающих с ним территориях РФ
Canters of Hygiene and Epidemiology of cities, regions, republics Центры гигиены и эпидемиологии городов, областей, республик | The number of samples examined for the presence of respiratory pathogens by RT-PCR Число образцов, изученных на наличие респираторных патогенов методом ОТ-ПЦР | |||||||||||
influenza viruses грипп | acute respiratory infections, seasonal ОРВИ, сезонные | SARS-CoV-2 | ||||||||||
number of samples число образцов | % «+» | number of samples число образцов | HPIV % «+» | HAdV % «+» | HRsV % «+» | HRV % «+» | HCoV % «+» | HBoV % «+» | HMPV % «+» | number of samples число образцов | % «+» | |
CEEI, Moscow ЦЭЭГ, Москва | 1404 | 13.8 | 595 | 1.5 | 3.4 | 2.7 | 12.9 | 3.7 | 0.2 | 2.0 | 1404 | 13.6 |
Vel. Novgorod Вел. Новгород | 1222 | 1.8 | 1024 | 0.7 | 0 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 |
Lipetsk Липецк | 4117 | 1.5 | 4117 | 0.5 | 0.3 | 0.3 | 0.5 | 0.4 | 0.6 | 4117 | 0.8 | |
Vladimir Владимир | 2330 | 5.7 | 1663 | 2.4 | 1.3 | 5.5 | 6.8 | 2.1 | 2.4 | 2.2 | 2234 | 3.2 |
Yaroslavl Ярославль | 6196 | 8.5 | 4159 | 1.7 | 2.3 | 2.7 | 8.3 | 3.9 | 1.0 | 4.1 | 4587 | 8.2 |
Penza Пенза | 4058 | 4.2 | 3549 | 1.1 | 1.6 | 2.3 | 5.0 | 1.1 | 0.4 | 0.5 | 3549 | 1.3 |
Cheboksary Чебоксары | 3348 | 6.2 | 3348 | 0.7 | 0.6 | 0.9 | 0.03 | 1.6 | 0 | 0 | 3422 | 10.0 |
Orenburg Оренбург | 10 817 | 1.7 | 10 817 | 1.5 | 1.0 | 2.1 | 4.6 | 1.5 | 0.8 | 2.6 | 0 | 0 |
Tomsk Томск | 3265 | 12.9 | 3254 | 1.32 | 1.5 | 2.9 | 8.8 | 2.1 | 0.8 | 1.2 | 3265 | 6.3 |
Vladivostok Владивосток | 5345 | 13.1 | 5345 | 1.9 | 1.8 | 4.7 | 10.5 | 4.0 | 1.0 | 3.1 | 5345 | 8.3 |
Birobidzhan Биробиджан | 2617 | 12.7 | 2406 | 2.9 | 2.5 | 1.1 | 12.6 | 4.2 | 0.9 | 2.5 | 2967 | 8.0 |
Total Всего | 44 719 | 6.6 | 40 277 | 1.5 | 1.3 | 2.4 | 5.9 | 2.1 | 0.8 | 2.0 | 30 890 | 6.3 |
The highest frequency of positive influenza samples was observed in Moscow, Vladivostok, Tomsk and Birobidzhan; SARS-CoV-2 – in Moscow, Cheboksary, Vladivostok and Yaroslavl. HRV (5.9%), HRSV (2.4%) and HCoV (2.1%) were considered the top three “leaders” in the structure of seasonal acute respiratory viral infections, with higher rates of positive samples for HPIV detected in Birobidzhan, Vladimir and Yaroslavl; for HAdV – in Moscow, Birobidzhan and Yaroslavl. Moscow, Birobidzhan and Yaroslavl; HRV – in Moscow, Birobidzhan and Vladivostok; HCoV – in Yaroslavl, Moscow and Vladimir; HBoV – in Vladimir, Vladivostok and Birobidzhan; HMPV – in Yaroslavl, Orenburg and Birobidzhan.
At the same time, the regions of Russia did not differ in the proportion of influenza virus types/subtypes (Fig. 2). Influenza A virus dominated in the 2023‒2024 season in all territories cooperating with the NRCEM CEEI. In the structure of influenza A virus, A(H3N2) was most active, with a share of 95.0%. Influenza B virus strains were detected only in 5.0% of cases, while in certain cities its activity was higher compared to other cities in the European part of the Russian Federation: in Moscow (10.3%), Lipetsk (15.3%), Vladimir (7.5%), Yaroslavl (5.5%) and Vladivostok (8.7%).
Fig. 2. The share of influenza viruses during the epidemic season 2023–2024 in different regions of the Russian Federation (according to the Centers of Hygiene and Epidemiology of cities, regions, republics).
Рис. 2. Долевое участие вирусов гриппа в период эпидемического сезона 2023–2024 гг. в разных регионах РФ (по данным Центров гигиены и эпидемиологии городов, областей, республик).
The results of antigenic characterization of epidemic strains are presented in Table 2. The studies were conducted with respect to influenza viruses included in the vaccines for the Northern Hemisphere countries in the 2023‒2024 season3.
Table 2. Antigenic properties of epidemic strains of influenza A and B viruses isolated in the epidemic season 2023–2024
Таблица 2. Антигенные свойства эпидемических штаммов вирусов гриппа А и В. выделенных в эпидемическом сезоне 2023–2024 гг.
Type/subtype of the influenza virus Тип/подтип вируса гриппа | Influenza virus strains included in influenza vaccines in the 2023–2024 season (relation to homologous titer) Штаммы вирусов гриппа. вошедшие в состав гриппозных вакцин в сезоне 2023–2024 гг. (отношение к гомологичному титру) | The number of strains closely related to the reference serum/the number of studied Число штаммов. близкородственных эталонной сыворотке/число изученных | Общее число изученных штаммов The total number of strains studied |
А(H1N1)pdm09 | A/Victoria/4897/22 А/Виктория/4897/22 (1–1/2 : 1/4) | 2 (100%) : 0 | 2 |
Drift variant Дрейф-вариант (< 1/4) | 0 | ||
А(H3N2) | A/Darwin/9/21 А/Дарвин/9/21 (1–1/2 : 1/4) | 52 (27.8%) : 103 (55.1%) | 187 |
Drift variant Дрейф-вариант (< 1/4) | 32 (17.1%) | ||
В | Victoria-like lineage Линия Виктория-подобных B/Austria/135941/21 (1–1/2) В/Австрия/135941/21(∆3) | 0 : 6 (46.2%) | 13 |
Victoria-like lineage Линия Виктория-подобных Drift variant Дрейф-вариант (< 1/4) | 7 (53.8%) |
During the period under review, the antigenic properties of 202 hemagglutinating isolates isolated in Moscow, Veliky Novgorod, Yaroslavl, Tomsk, Orenburg, Birobidzhan and Vladivostok were studied with a spectrum of specific reference sera. 187 isolates showed affinity to influenza A(H3N2) virus, and 17.1% of them were drift variants and interacted with serum to vaccine virus A/Darwin/9/21(H3N2) to 1/8 and below the homologous titer; two isolates showed close affinity to influenza A(H1N1)pdm09; 13 isolates were related to influenza B virus and interacted with serum to vaccine virus B/Austria/1359417/21 from 1/16 to 1/4 of the homologous titer.
The sensitivity of epidemic strains of influenza viruses isolated in different cities of the Russian Federation to drugs with antineuraminidase activity was studied. The data are presented in Table 3.
Таблица 3. Чувствительность эпидемических штаммов вирусов гриппа А и В, выделенных в сезоне 2023–2024 гг., к препаратам с антинейраминидазной активностью
Table 3. The susceptibility of influenza epidemic strains A and B, isolated in season 2023–2024 to inhibitors of neuraminidase
ФБУЗ «Центр гигиены и эпидемиологии» Роспотребнадзора Center for Hygiene and Epidemiology of Rospotrebnadzor | Число штаммов Number of strains | Штаммы вируса гриппа Influenza virus strains | IC50 (nM) к антинейраминидазным препаратам (интервалы среднего значения) IC50 (nM) to antineuraminidase drugs (mean value intervals) | |
осельтамивиру oseltamivir | занамивиру zanamivir | |||
ЦЭЭГ, НИЦЭМ, Москва NRCEM CEEI, Moscow | 2 | A(H1N1)pdm09 | 0,5–0,7 | 0,4–0,9 |
56 | A(H3N2) | 0,3–5,0 | 0,9–8,3 | |
3 | B | 35,6–38,9 | 4,5–5,1 | |
Новгородской области / Novgorod region | 2 | A(H3N2) | 0,9–3,1 | 1,9–4,3 |
Ярославской области / Yaroslavl region | 2 | A(H3N2) | 0,5–0,6 | 0,5–1,6 |
Оренбургской области / Orenburg region | 10 | A(H3N2) | 0,3–0,9 | 0,6–4,2 |
Томской области / Tomsk region | 5 | A(H3N2) | 0,4–0,6 | 0,9–1,2 |
Приморского края / Primorsky Krai | 10 | A(H3N2) | 0,4–0,7 | 0,8–2,0 |
4 | B | 22,0–42,7 | 2,0–7,3 | |
Еврейской АО / Jewish Autonomous District | 4 | A(H3N2) | 0,4–1,0 | 0,8–4,1 |
ИТОГО TOTAL | 2 | A(H1N1)pdm09 | 0,5–0,7 | 0,4–0,9 |
89 | A(H3N2) | 0,3–5,0 | 0,5–8,3 | |
7 | В | 22,0–42,7 | 2,0–7,3 | |
All of the tested strains showed normal sensitivity to oseltamivir and zanamivir, except for 2 influenza A(H3N2) strains, A/Moskva/26/2023 and A/Moskva/3/2024 (H3N2), which showed reduced sensitivity to oseltamivir and zanamivir.
Whole genome sequences for 62 strains of influenza A viruses, including 58 strains of A(H3N2) and 4 strains of A(H1N1)pdm09 isolated in different regions of the Russian Federation, were obtained by high-throughput sequencing.
Influenza A(H3N2) viruses were assigned to clade 3C.2a1b.2a.2a.2a.3a.1 (2a.3a.1), represented by virus A/Thailand/8/2022. In 72.0% of the population, common substitutions N122D, K276E, characteristic of subclade J.2 (reference strain A/ Sydney/878/2023) were detected; furthermore, 12 strains were identified that also carried additional mutations in HA such as I25V, V347M, more characteristic of subclade J.1 (reference strain A/ Sydney/856/2023); in the Far East, original strains (13) carrying additional F79L, P239S substitutions in HA were identified in relation to the J.2 clade: (EPI_ISL_18807375, EPI_ISL_18808360, EPI_ISL_18808361, EPI_ISL_18808362, EPI_ISL_18808363, EPI_ISL_18808364, EPI_ISL_18808365, EPI_ISL_18808371, EPI_ISL_19072582, EPI_ISL_19072580, EPI_ISL_19072579, EPI_ISL_19072578, EPI_ISL_19072577, EPI_ISL_19072576, EPI_ISL_19072575, EPI_ISL_19072574, EPI_ISL_19072573, EPI_ISL_19072572, EPI_ISL_19072571, EPI_ISL_19072570, EPI_ISL_19072569, EPI_ISL_19072524, EPI_ISL_19072523, EPI_ISL_19072522, EPI_ISL_19072521, EPI_ISL_19072520, EPI_ISL_19072519, EPI_ISL_19072518, EPI_ISL_19072517, EPI_ISL_19072516, EPI_ISL_19072515, EPI_ISL_19072514, EPI_ISL_19072513, EPI_ISL_19072512, EPI_ISL_19072511, EPI_ISL_19072510, EPI_ISL_19072509, EPI_ISL_19072508, EPI_ISL_19072507, EPI_ISL_19072506, EPI_ISL_19072505, EPI_ISL_19072504, EPI_ISL_19072503, EPI_ISL_19072502, EPI_ISL_19072501, EPI_ISL_19072500, EPI_ISL_19072499, EPI_ISL_19072498, EPI_ISL_19072497, EPI_ISL_19072496, EPI_ISL_19072495, EPI_ISL_19072494, EPI_ISL_19072493, EPI_ISL_19072492, EPI_ISL_19072491, EPI_ISL_19072490, EPI_ISL_19072489, EPI_ISL_19072488).
Influenza A(H1N1)pdm09 influenza virus strains (4) were closely related to the vaccine virus A/Victoria/2570/19, represented by clade 6B.1A.5a.2a (EPI_ISL_18809332, EPI_ISL_18809333, EPI_ISL_18809334, EPI_ISL_18809335).
No genetic markers responsible for decreased sensitivity to neuraminidase (oseltamivir, zanamivir) and polymerase complex (baloxavir marboxil) inhibitors were identified among the strains studied.
Discussion
An early increase in influenza virus activity against a background of relatively low activity and continued evolutionary variability of SARs-CoV-2 was observed during the period under review in most countries of the Northern Hemisphere4,5,6.
According to WHO, from October 1, 2023 to June 30, 2024, more than 10 million clinical specimens were tested worldwide, of which 12.3% were positive for influenza viruses. An increase in the detection rate of positive samples for influenza viruses was noted from the beginning of October 2023, with the detection rate exceeding the 10% threshold during week 47 of 2023 (11.0%); the highest rates were recorded during the last week of 2023 (33.0%); a decrease in this rate started from week 14 of 2024; by the end of the season (week 39 of 2024) it was 4.0%, which was higher compared to the previous season (1.4%). During the period analyzed, the proportion of influenza viruses was distributed as follows: influenza type A, 78.0%, and influenza type B, 22.0%; 25.0% among the subtyped influenza A viruses were assigned to A(H1N1)pdm09 and 75.0% to A(H3N2); all of the subtyped influenza B viruses were assigned to the B/Victoria-like lineage. Differences in the proportion of influenza viruses by country, temperature zone and WHO region were observed, as in previous seasons.
In countries of the WHO European Region, increasing rates of positive samples for respiratory pathogens were associated with influenza A(H3N2) virus, with a maximum in week 5 of 2024 (26.1%); decreasing during week 9 of 2024 (9.8%)5. Influenza B virus was detected from the 13th week of 2024, mostly in sporadic cases. During the period under review, the proportion of cases was distributed as follows: influenza type A – 43741 (93.4%) and influenza type B – 3066 (6.4%); 10.4% among influenza A subtyped viruses were A(H1N1)pdm09 and 89.6% were A(H3N2); all of the influenza B subtyped viruses (159) were assigned to the B/Victoria-like lineage.
In countries of the WHO Region of the Americas, the duration of the influenza epidemic was 15 weeks (from week 49 of 2023), with peak activity in late December 2023 (18.1%), with differences in the predominant virus type: in North America, influenza virus type A(H1N1)pdm09 dominated (76.5% of the influenza A virus strains typed)6; in Central America and the Caribbean, A(H3N2) dominated, with a frequency of positive samples of up to 81.0%.
Countries in the WHO South-East Region recorded relatively low influenza virus activity (up to 10.0% of positive samples tested), with almost equal activity detected for all 3 influenza viruses.
Countries in the WHO Western Pacific Region showed high A(H3N2) activity, with a peak in the number of positive samples at week 49 (34.1%); from week 10 of 2024, an increase in A(H1N1)pdm09 influenza virus activity was recorded, with a peak in week 13 of 2024.
In countries in the WHO African Region, co-circulation of influenza A viruses was recorded from week 40 of 2023, with high activity of A(H1N1)pdm09 during certain weeks of the season, while influenza B virus was detected only in sporadic cases. Rates above the threshold (10.0%) were recorded from week 40 of 2023 to week 3 of 2024 and from week 18 to week 26 of 2024 (up to 250 positive samples per week). In central African countries, the proportion of influenza viruses was: for influenza A, 62.0% with equal participation of both subtypes; influenza B, 38.0%. In eastern African countries, the proportion of influenza viruses was: for influenza A, 87.0%, including influenza A(H3N2), 72.0%; influenza B, 13.0%.
In countries in the WHO Eastern Mediterranean Region, the start of the season was marked by relatively high rates of positive samples for influenza viruses (22.7% during week 40 of 2023), peaking by week 43 of 2023 (29.1%). During this period, high activity of influenza A(H1N1)pdm09 and B viruses was observed in several countries in Bahrain, Egypt and Lebanon; co-circulation of A(H3N2) and B was recorded in Afghanistan; A(H1N1)pdm09 and A(H3N2) in Tunisia.
A study of the genetic properties of the population of circulating strains revealed an incomplete correspondence with the strains included in influenza vaccines in the 2023‒2024 season for the countries of the Northern Hemisphere.
The population of circulating influenza A(H1N1)pdm09 strains was represented by variants of clade 5a.2a (subclades C.1, C.7, C.1.7.2, C.8, C.1.9) and clade 5a.2a.1 (subclades C.1.1, D, D.1, D.2, D.3, D.4), comprising the references – A/Victoria/2570/2017 (vaccine), A/Sydney/5/2021, A/Wisconsin/67/2022. The highest activity was observed for subclade C.1.9, which was dominant in most regions, except in North America, Central and South America, where subclade D strains were prevalent.
Influenza A(H3N2) virus strains were assigned to clade 3C.2a1b.2a.2a.2a.3a.1 (subclades J.1‒J.4; A/Thailand/18/2022), dominated by subclade J.2., whose representatives had specific substitutions in hemagglutinin (HA) – N122D and R276E. Subclade J.1 strains (I25V, V347M) co-circulated with low frequency in many countries, except in Asia and Africa, where their prevalence was noted. Subclades G.1.3.1 (2a.3a) and J.4 (2a.3a.1) circulated in several countries in West Africa.
Influenza B viruses were less active and all of the typed viruses belonged to the B/Victoria-like lineage, clade V1A.3a.2 (B/Austria/1359417/2021); representatives of the B/Yamagata-like lineage were not detected.
Taking into account the peculiarities of the properties of the circulating strains, WHO experts substituted influenza vaccines with the influenza A(H3N2) virus component: A/Darwin/9/2021 (clade 2a) to A/Thailand/8/2022 (clade 2a.3a.1, subclade J). It should be noted that in the period February‒September 2024, population heterogeneity of influenza virus strains continued to be registered in the population, and to a greater extent it affected influenza virus A(H3N2)pdm09 (predominant in Russia and European countries), which became the reason for its replacement in the composition of influenza vaccines for countries of the Southern Hemisphere with a more relevant one (A/Thailand/8/2022, clade 2a.3a.1, subclade J to A/Croatia/10136RV/2023, clade).
According to WHO data from the 2023‒2024 season, the sensitivity of more than 15,000 influenza A and B virus strains to drugs with antineuraminidase activity and about 10,000 to baloxavirus marboxil was tested. The results showed a good sensitivity profile to all drugs; in general, reduced sensitivity to drugs with antineuraminidase activity was determined among A(H1N1)pdm09 strains in 1.6%, A(H3N2) in 0.01% and influenza B in 0.2%; to baloxavir marboxil in 0.1, 0.3 and 0%, respectively.
The second respiratory pathogen for which countries conduct international monitoring is the new coronavirus SARS-CoV-21. In the epidemic season under review, its activity was significantly lower compared to previous seasons (in general, the frequency of positive samples did not exceed 10.0%, with the exception of summer months, during which maximum rates of up to 13.0% were recorded), and its highest activity was observed in countries of the WHO European Region. A total of 776,301,484 cases of SARS-CoV-2 infection had been reported worldwide by September 15, 2024, of which 7,066,329 (0.9%) were fatal. The top three countries by number of infections were Europe (36.0%), the Pacific (27.0%) and the Americas (25.0%). At the same time, the top three countries in terms of the frequency of fatal outcomes were Europe (32.0%), America (43.0%) and South-East Asia (11.0%). The dependence of SARS-CoV-2 activity on the activity of influenza viruses was traced; it seems that unlike previous years, SARS-CoV-2 activity decreases against the background of high activity of influenza viruses. It can be assumed that SARS-CoV-2 has occupied its niche in the structure of classical acute respiratory infections.
Furthermore, new variants were also observed in this virus population with dominance in certain periods. At the beginning of the season (October 2023), variants XBB 1.5+F456 (64.5%), BA.2 (20.0%) and BA.2.75 (8.0%) were the most frequently detected. In the following months, the BA.2.86 variant was observed to increase (from November 2023), and by January 2024 its percentage was 92.0%. New BA.2.86 variants entered the circulation, in particular JN.1, whose variability was recorded in the following months. JN.1 became the “progenitor” of the strain population named FLiRT. In the period of September 2024, among the most common subclades of FLiRT in the European region were such representatives as KP.3.1.1 (45%), XEC (13%), KP.3.1 (11%), JN.1 (9%), JN.1.11 (3%), JN.1.16 (3%), JN.1.9 (3%), KP.2 (3%)5.
A.A. Sominina and coauthors reported changes in the etiologic structure of moderate and severe acute respiratory viral infections during the COVID-19 pandemic, in particular, a significant decrease in the detection rate of influenza and HRSV viruses (2020‒2021), an increase in the number of cases of metapneumovirus and rhinovirus infections; an increase in the specific weight of HRSV infection in children against the background of a significant decrease in the frequency of COVID-19 (2022‒2023) [3]. In intensive care units, HRSV infection was most often detected in children in the post-pandemic period, while SARS-CoV-2 was most often detected in adults. The data obtained in this study confirm to a certain extent the previously identified trends.
A particularly concerning fact is the ability of avian and swine influenza viruses to infect humans, often resulting in severe cases of disease7 [10]. Among the most pathogenic are influenza A viruses with hemagglutinin subtypes H5, H7 and H9.
In the period between September 2023 and August 2024. in some countries of the world infection with avian influenza A(H5N1) virus was confirmed in 23 cases (Cambodia – 14, USA – 6, 1 case each in China, Australia and Vietnam), A(H5N6) – 5 cases (China), A(H9N2) – 14 cases (China – 11, 1 case each in Ghana, India and Vietnam), A(H10N3) – 1 case (China), A(H10N5) – 1 case (China); infection with swine influenza viruses A(H1N1)v – 5 cases (USA, Vietnam, Brazil, Spain, Switzerland, A(H1N2)v – 5 cases (USA – 4. UK – 1), A(H3N2)v – 1 case (Canada)8.
Since 2024, cases of infection of farmers with avian influenza A(H5N1) virus from cows (clade 2.3.4.4.4.b, genotype B3.13) and from birds on poultry farms (genotype D1.1)6 have been reported in the United States. At the same time, no cases of human-to-human transmission have been registered since 2007. This probably correlates with data from experiments on ferrets, where it was shown that the virus of clade 2.3.4.4.b, including genotype B3.13, was transmitted quite well by direct contact and extremely limitedly by airborne transmission [11]. Current virological and epidemiological data show that despite the ability of avian influenza viruses to infect humans, they remain avian in their properties with no “signs” of adaptation to mammals, although the events of 2024 in the USA claim the opposite: avian influenza virus A(H5N1) was able to infect cows and was then transmitted to humans. All of the above mentioned confirms the necessity for further research and monitoring.
Conclusion
The epidemic season of 2023‒2024 was characterized by a relatively low activity of SARS-CoV-2 and its new variants. The epidemic season of 2023‒2024 had its own peculiarities and, in particular, against the background of relatively low activity of SARS-CoV-2 and its new variants, was characterized by an earlier onset, the highest activity of influenza A virus, with the countries of the world differing in the dominant subtype (A(H1N)pdm09 or A(H3N2)), as well as in the proportion of influenza B lineage B/Victoria-like virus; influenza B/Yamagata-like virus has not been active since March 2020. Depending on the activity of the type/subtype of influenza virus, differences in the incidence, involvement of age groups, and lethality were noted at certain periods of the season. By antigenic and molecular genetic properties, the population of epidemic strains of influenza viruses was close to the viruses included in the influenza vaccines recommended by WHO experts in the season 2022‒2023, which suggests their high efficacy; a favorable sensitivity profile to drugs with antineuraminidase activity, as well as an inhibitor of the enzyme that synthesizes the matrix RNA of influenza virus was preserved. HRV (5.9%), HRSV (2.4%) and HCoV (2.1%) represented the top three “leaders” in the structure of seasonal acute respiratory viral infections. Cases of human infection with avian and swine influenza viruses continue to be registered in the countries of the world. All of the above mentioned confirms the relevance of research and data obtained as part of ongoing surveillance for influenza virus circulation.
1 COVID-19 epidemiological update – 9 October 2024. Edition 172. Available at: https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-172
2 Risk assessments and summaries of influenza at the human-animal interface. Available at: https://www.who.int/teams/global-influenza-programme/avian-influenza/monthly-risk-assessment-summary.
3 Recommendations announced for influenza vaccine composition for the 2024-2025 northern hemisphere influenza season; 2024. Available at: https://www.who.int/ru/news/item/23-02-2024-recommendations-announced-for-influenza-vaccine-composition-for-the-2024-2025-northern-hemisphere-influenza-season
4 Global influenza programme. Influenza updates. Available at: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-updates
5 Seasonal influenza - Annual Epidemiological Report for 2023/2024. Available at: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-annual-epidemiological-report-20232024
6 Weekly U.S. Influenza Surveillance Report. Available at: http://www.cdc.gov/flu/weekly/index.htm/.
7 Recommendations announced for influenza vaccine composition for the 2025 southern hemisphere influenza season; 2024. Available at: https://www.who.int/ru/news/item/27-09-2024-recommendations-announced-for-influenza-vaccine-composition-for-the-2025-southern-hemisphere-influenza-season
8 Influenza (avian and other zoonotic). Available at: https://www.who.int/health-topics/influenza-avian-and-other-zoonotic#tab=tab_1
About the authors
Elena I. Burtseva
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Author for correspondence.
Email: elena-burtseva@yandex.ru
ORCID iD: 0000-0003-2518-6801
ScD, Head of influenza etiology and epidemiology laboratory
Russian Federation, 123098, MoscowNataliya V. Breslav
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: n.belyakova1983@gmail.com
ORCID iD: 0000-0002-6946-5119
PhD, Senior Researcher
Russian Federation, 123098, MoscowEvgenia A. Mukasheva
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: mukasheva_evgeniya@mail.ru
ORCID iD: 0000-0002-5688-5309
Research Associate
Russian Federation, 123098, MoscowKirill G. Krasnoslobodtsev
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: kkg_87@mail.ru
ORCID iD: 0000-0003-1745-9128
Research Associate
Russian Federation, 123098, MoscowElena S. Kirillova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: esshevchenko@yandex.ru
ORCID iD: 0000-0001-7977-7530
PhD, Leading Researcher
Russian Federation, 123098, MoscowSvetlana V. Trushakova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: s.trushakova@gmail.com
ORCID iD: 0000-0002-9610-3041
PhD, Senior Researcher
Russian Federation, 123098, MoscowIrina A. Komarova
Pacific State Medical University of the Ministry of Health of the Russian Federation
Email: mikhaira@yandex.ru
ORCID iD: 0000-0003-0483-7433
Assistant of the Department of Infectious Diseases
Russian Federation, 690002, Primorsky Krai, VladivostokElena L. Feodoritova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: flulab@mail.ru
ORCID iD: 0000-0002-1472-1357
Research Associate
Russian Federation, 123098, MoscowAnna D. Panova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: ainushgnomello@gmail.com
ORCID iD: 0000-0002-9322-6273
junior research assistant
Russian Federation, 123098, MoscowLidiya B. Kisteneva
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: lborisovna2007@yandex.ru
ORCID iD: 0000-0001-7336-409X
head of laboratory
Russian Federation, 123098, MoscowIrina N. Khlopova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: khlopova.ira@yandex.ru
ORCID iD: 0000-0002-7419-590X
PhD, Leading Researcher
Russian Federation, 123098, MoscowIrina S. Kruzhkova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: irina-kru@yandex.ru
ORCID iD: 0000-0002-1983-481X
junior research assistant
Russian Federation, 123098, MoscowAnastasia S. Krepkaia
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: nastya18-96@mail.ru
ORCID iD: 0000-0002-7272-4011
junior research assistant
Russian Federation, 123098, MoscowEkaterina O. Morozova
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: eo-morozova@inbox.ru
ORCID iD: 0009-0001-3367-6168
PhD, Senior Researcher
Russian Federation, 123098, MoscowAnna V. Ignatieva
The N.F. Gamaleya Research Center of Epidemiology and Microbiology of Ministry of Health
Email: valgella@yandex.ru
ORCID iD: 0000-0001-6206-2299
PhD, Senior Researcher
Russian Federation, 123098, MoscowAndrey B. Komissarov
Research institute of influenza named after A.A. Smorodintsev of Ministry of Health
Email: andrey.komissarov@influenza.spb.ru
ORCID iD: 0000-0003-1733-1255
Head of the Laboratory of Molecular Virology
Russian Federation, 197022, St. PetersburgIgor N. Tyurin
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: tyurin.dti@yandex.ru
ORCID iD: 0000-0002-5696-1586
PhD, Chief Physician
Russian Federation, 125367, MoscowAleksey A. Samkov
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: a.a.samkov@yandex.ru
ORCID iD: 0000-0002-0365-3096
Deputy Chief Medical Officer
Russian Federation, 125367, MoscowNataliya A. Antipjat
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: natadoc70@bk.ru
ORCID iD: 0000-0001-8578-2838
Deputy Chief Medical Officer
Russian Federation, 125367, MoscowReferences
- Burtseva E.I., Kolobukhina L.V., Voronina O.L., Ignatjeva A.V., Mukasheva E.A., Panova A.D., et al. Features of the circulation of ARVI pathogens during of emergence and widespread of SARS-CoV-2 in the 2018–2021. Epidemiologiya i Vaktsinoprofilaktika. 2022; 21(4): 16–26. https://doi.org/10.31631/2073-3046-2022-21-4-16-26 https://elibrary.ru/rnyfoi (in Russian)
- Burtseva E.I., Kolobukhina L.V., Panova A.D., Mukasheva E.A., Krasnoslobodtsev K.G., Kirillova E.S., et al. Properties of influenza viruses that caused epidemic increases in morbidity in Russia and countries of the world during 2022–2023. The effectiveness of vaccine prophylaxis. The effectiveness of vaccine prophylaxis. Voprosy virusologii. 2024; 69(1): 42–55. https://doi.org/10.36233/0507-4088-211 https://elibrary.ru/zqtfnx (in Russian)
- Sominina A.A., Danilenko D.M., Komissarov A.B., Pisareva M.M., Musaeva T.D., Stolyarov K.A., et al. Changes in the etiological structure of severe acute respiratory viral infections in children and adults under the influence of the COVID-19 pandemic. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2024; 101(3): 327–41. https://doi.org/10.36233/0372-9311-532 https://elibrary.ru/jmtwkj (in Russian)
- Petrova P.A., Konovalova N.I., Boyarintseva A.Y., Danilenko D.M., Vasilieva A.D., Shelepanova T.N., et al. Etiologic characteristics of influenza epidemics on the territory of Russia in the period of pandemic COVID-19 in 2020–2023. Epidemiologiya i Vaktsinoprofilaktika. 2024; 23(3): 88–97. https://doi.org/10.31631/2073-3046-2024-23-3-88-97 https://elibrary.ru/ofrzhj (in Russian)
- Karpova L.S., Pelikh M.Yu., Popovtseva N.M., Stolyarova T.P., Volik K.M., Stolyarov K.A. Coronavirus infection caused by the omicron variant and its daughter genovariants in Russia (2022–2023). Epidemiologiya i Vaktsinoprofilaktika. 2024; 23(2): 36–49. https://doi.org/10.31631/2073-3046-2024-23-2-36-49 https://elibrary.ru/uqdnmi (in Russian)
- Karpova L.S., Komissarov A.B., Stolyarov K.A., Popovtseva N.M., Stolyarova T.P., Pelikh M.Yu., et al. Features of the COVID-19 Epidemic Process in Each of the of the Five Waves of Morbidity in Russia. Epidemiologiya i Vaktsinoprofilaktika. 2023; 22(2): 23–36. https://doi.org/10.31631/2073-3046-2023-22-2-23-36 https://elibrary.ru/udxfrp (in Russian)
- Pshenichnaya N.Yu., ed. Influenza and Acute Respiratory Viral Infections in the XXI Century: A Guide for Doctors [Gripp i ORVI v XXI veke: rukovodstvo dlya vrachei]. Moscow: GEOTAR-Media; 2024. https://doi.org/10.33029/9704-8433-3-IAR-2024-1-304 (in Russian)
- Zhou B., Lin X., Wang W., Halpin R.A., Bera J., Stockwell T.B., et al. Universal influenza B virus genomic amplification facilitates, sequencing, diagnostics, and reverse genetics. J. Clin. Microbiol. 2014; 52(5): 1330–7. https://doi.org/10.1128/jcm.03265-13
- Zhou B., Donnelly M.E., Scholes D.T., St George K., Hatta M., Kawaoka Y., et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza A viruses. J. Virol. Oct. 2009; 83(19): 10309–13. https://doi.org/10.1128/jvi.01109-09
- Charostad J., Rezaei Zadeh Rukerd M., Mahmoudvand S., Bashash D., Hashemi S.M.A., Nakhaie M., et al. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel Med. Infect. Dis. 2023; 55: 102638. https://doi.org/10.1016/j.tmaid.2023.102638
- Belser J.A., Sun X., Pulit-Penaloza J.A., Maines T.R. Fatal infection in ferrets after ocular inoculation with highly pathogenic avian influenza A(H5N1) virus. Emerg. Infect. Dis. 2024; 30(7): 1484–7. https://doi.org/10.3201/eid3007.240520
Supplementary files