Occult hepatitis B: prevalence and clinical significance. Role in liver pathology and in viral coinfections
- Authors: Kushch A.A.1
-
Affiliations:
- N.F. Gamaleya National Research Centre for Epidemiology and Microbiology, Ministry of Health of the Russian Federation
- Issue: Vol 70, No 4 (2025)
- Pages: 299-316
- Section: REVIEWS
- URL: https://virusjour.crie.ru/jour/article/view/16714
- DOI: https://doi.org/10.36233/0507-4088-291
- EDN: https://elibrary.ru/gmsjsl
- ID: 16714
Cite item
Abstract
The review examines issues related to occult hepatitis B virus infection (OBI), which occurs at a late stage of chronic hepatitis B (CHB) after HBsAg clearance. In clinical practice, OBI is detected by the absence of HBsAg and the presence of antibodies to HBcAg in the blood serum and is often referred to as «past» or «resolved» hepatitis B. However, hepatitis B virus (HBV) DNA remains in liver cells, is poorly detected by routine diagnostic methods, and cannot be removed by existing therapies. Data on the prevalence of OBI vary, but it is found in all regions of the world, much more often in regions with a high prevalence of HBV. Data on the association of OBI with fibrosis, cirrhosis and hepatocellular carcinoma (HCC) have been obtained. It has been established that OBI is associated with an increased risk of HBV reactivation in patients with infections with other viruses, as well as in cancer patients whose treatment includes immunosuppressive therapy. HBV reactivation leads to severe consequences and, in the absence of treatment, death of patients. It can be concluded that to achieve the goal set by WHO for the eradication of viral hepatitis by 2030, it is necessary to solve the problem of OBI. In order to make this possible, it is essential to create new, more sensitive and informative diagnostic tests, effective methods of HBV DNA elimination, and to investigate the mechanisms of OBI development in more depth.
Full Text
Introduction
The hepatitis B virus (HBV) can cause acute and chronic liver diseases that pose a potential threat to life. According to the World Health Organization (WHO), published in 2024, 254 million people are chronically infected with HBV, with 1.2 million new cases of infection and 1.1 million deaths annually, mainly from liver cirrhosis and primary liver cancer[1], and these statistics could increase by 2030 [1]. For over four decades, a safe and effective vaccine against hepatitis B has been in use; however, the concentration of antiviral antibodies after vaccination gradually decreases to a level below protective, and certain people do not respond to vaccination, which contributes to the emergence of new cases of HBV and the chronicization of the infection. To understand the causes of this phenomenon, a more in-depth study of the virus, its life cycle, and the diseases it causes is necessary.
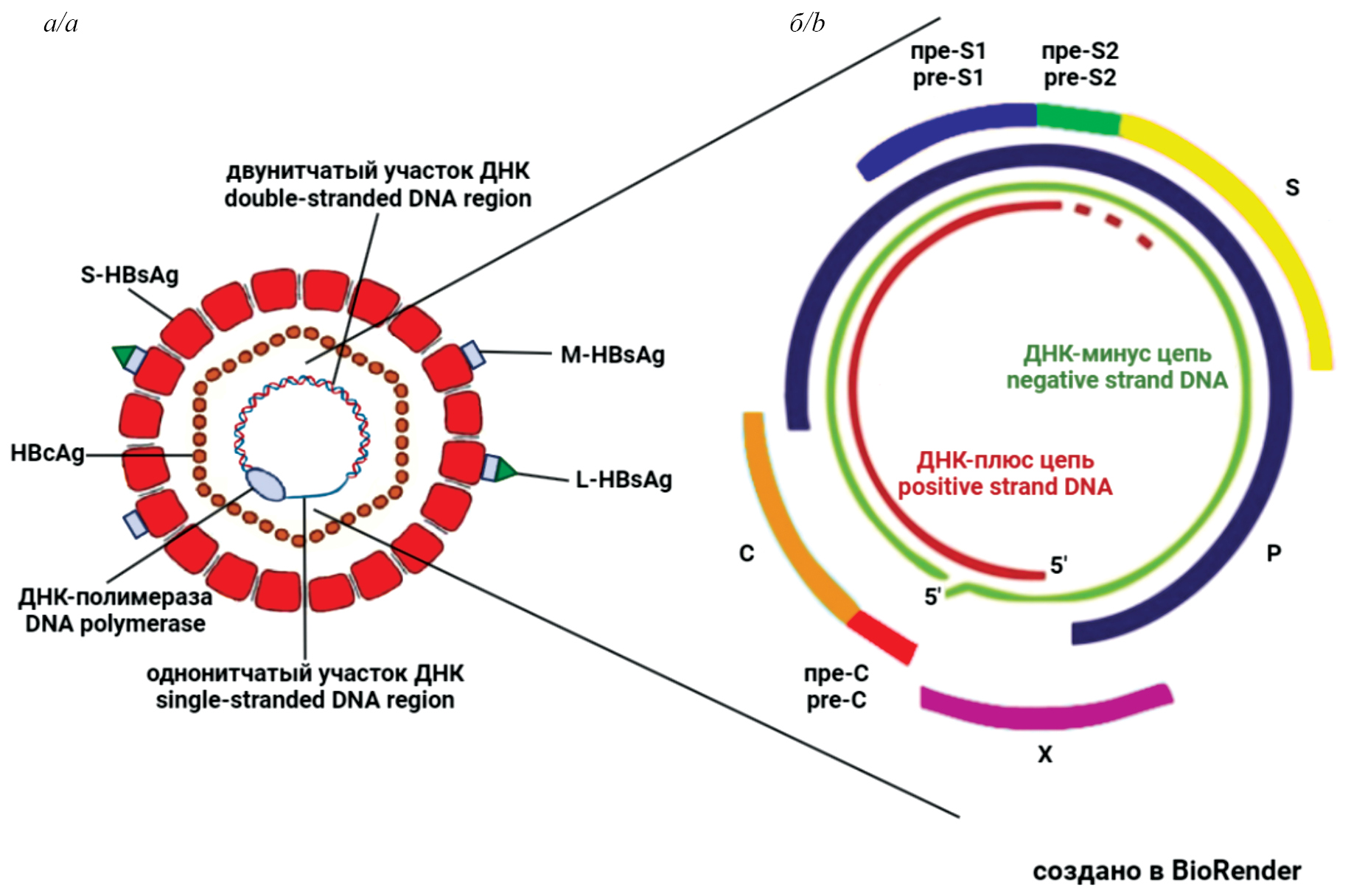
The HBV virion (Dane particle) consists of a lipid envelope containing large (L), medium (M) and small (S) surface proteins, which represent the HBV surface antigen – HBsAg. Inside the virion is a nucleocapsid composed of 120 dimers of the core protein C (core) – HBc (HBcAg), containing viral DNA and covalently attached polymerase (P) of HBV (Fig. 1 a) [2, 3]. Moreover, the HBV genome encodes a non-structural regulatory protein X, which is found both in the nucleus and in the cytoplasm of infected cells [4, 5]. The HBV genome (approximately 3.2 kb) is represented by partially double-stranded relaxed circular DNA, containing four partially overlapping open reading frames (ORF): PreS1/PreS2/S, PreC/C, P and X, which serve as templates for the formation of four HBV proteins – S, C, P and X (Fig. 1 b).
Fig. 1. Schematic representation of the hepatitis B virus structure.
a – the Dane particle – the HBV virion; b – the viral genome.
Рис. 1. Схематическое изображение структуры вируса гепатита В.
а – частица Дейна – вирион ВГВ; б – вирусный геном.
The life cycle of HBV is schematically presented in Fig. 2. The virus, having entered the body, reaches the liver and attaches to the hepatocyte membrane, for which the preS1 domain interacts with heparan sulfate – a nonspecific receptor on the surface of hepatocytes. Then the virus binds with high affinity to the cellular receptor for HBV – NTCP (sodium(Na+) taurocholate cotransporting polypeptide) [6]. Recently, it has been shown that the epidermal growth factor receptor also plays an important role in the entry of the HBV virion into the cell [7]. After internalization via endocytosis, the virus loses its envelope (uncoating), and the nucleocapsid particles (core), containing relaxed circular DNA (rcDNA) and viral polymerase, are transported to the nucleus. In the nucleoplasm, cellular enzymes repair rcDNA and convert it into covalently closed circular DNA (cccDNA). cccDNA associates with histones and non-histone proteins to form a mini-chromosome [8]. cccDNA is transcribed by cellular RNA polymerase II, resulting in a set of viral mRNA, including pregenomic RNA (pgRNA). Viral transcripts are then transported to the cytoplasm and translated into 7 HBV proteins: e-antigen (HBeAg), core (HBc), polymerase (Pol), 3 surface proteins (large – L-HBs, medium – M-HBs, small – S-HBs) and protein X (HBx). Core proteins oligomerize around the viral polymerase and pgRNA, and as a result of the encapsidation reaction, a capsid is formed. In the capsid involving Pol, reverse transcription of pgRNA into rcDNA occurs, initially synthesizing the complementary negative single-stranded DNA, followed by the second positive strand of DNA. Mature capsids containing rcDNA are then transported to the endoplasmic reticulum (ER), where they acquire surface proteins. The virions formed in this manner exit the infected cell via exocytosis and are able to infect new cells. Certain capsids containing rcDNA can be transported back to the nucleus to maintain the pool of cDNA (Fig. 2) [9, 10].
Fig. 2. Schematic diagram of the hepatitis B virus life cycle in an infected cell.
Explanations in the text.
Рис. 2. Схематическое представление жизненного цикла вируса гепатита В в инфицированной клетке.
Пояснения в тексте.
Along with the formation and maintenance of episomal cccDNA in the nucleus, which is necessary for viral replication, HBV DNA integration into the host genome often occurs at an early stage of infection [11, 12]. The integrated genome is incapable of replication, while certain genes remain active, and data is accumulating on the role of integrated DNA in the development of liver cancer [13, 14].
Perinatal HBV infection is a serious disease, with 90% of infected infants progressing from acute to chronic infection [15]. Acute HBV infection in adult patients is characterized in most cases by spontaneous resolution [16], during which HBsAg disappears from peripheral blood (PB), viral DNA concentration decreases, and there are no serological, biochemical, or clinical signs of hepatitis. In such cases, it is referred to as a resolved infection.
T.I. Michalak et al. were the first to show that after recovery from acute hepatitis B, HBV DNA can be persistently (up to 70 months) detected in the serum and peripheral blood mononuclear cells. The authors concluded that the disappearance of HBsAg from the serum, the production of anti-HBs antibodies, and even the normalization of liver function may not reflect complete recovery from an infection caused by HBV [17]. This conclusion has been confirmed by numerous subsequent studies, which have shown that modern hepatitis B treatments do not ensure complete eradication of the virus [18, 19]. This is related to the peculiarities of the virus’s life cycle and the dynamics of the infectious process.
Immediately after HBV infection, the virus’s DNA and HBsAg may not be detectable in the serum/plasma, but they can still be transmitted to recipients. An example is described in the study [20], in which the authors examined two blood donors and did not detect either HBsAg or HBV DNA. However, hepatitis B virus (HBV) was detected in the recipients of blood from these donors. Both donors subsequently tested positive for HBsAg and developed acute hepatitis. According to other authors [21], the diagnostic window period can range from 19 to 35 days. To support their findings, the authors provided data indicating that 35 days after receiving material from the donor, neither HBsAg nor HBV DNA were detected in it; however, the recipient developed an HBV infection with a virus strain identical to the strain that was later detected in the donor. Similar data are provided by I.F. Golubeva et al., who concluded that HBsAg is detected in most cases following the incubation period 3–5 weeks after the moment of infection [22]. It is believed that during the window period, which is before HBV DNA appears in the blood, it is most difficult to detect the infection.
The hidden (occult) period, undetectable by routine testing, can occur both in the early and the later stage of the infection (Fig. 3).
Fig. 3. Changes in the levels of hepatitis B serological markers in the dynamics of HBV infection.
Explanations in the text.
Рис. 3. Изменения уровней серологических маркеров гепатита В в динамике ВГВ-инфекции.
Пояснения в тексте.
HBsAg is an early serological marker of HBV infection, the level of which significantly increases upon entering the active phase, which is also characterized by a high level of HBV DNA replication, synthesis, and the appearance of HBsAg and HBeAg in the blood plasma (Fig. 3). The detection of HBsAg in the blood 6 months or more after infection indicates the transition of the infection to the chronic stage, during which seroconversion occurs with the appearance of antiviral antibodies in the blood: anti-HBs, anti-HBc, anti-HBe [23]. Chronic hepatitis B can last for years.
With the advent of HBV DNA detection methods in the 1980s, data accumulated showing that HBsAg-negative [HBsAg(−)] hepatitis occurs both in endemic regions where chronic hepatitis B is prevalent (> 8%) and in regions with relatively low chronic hepatitis B prevalence (< 2%) [24]. Chronic hepatitis in patients with HBsAg(−) but with HBV DNA(+) has come to be called hidden or occult.
By 2017, numerous data on HBsAg(−) hepatitis led the European Association for the Study of the Liver (EASL) to revise the classification of chronic hepatitis, which previously included 4 stages of infection. The stages/phases of chronic hepatitis B were designated as follows: I – HBeAg-positive chronic infection, II – HBeAg-positive chronic hepatitis, III – HBeAg-negative chronic infection, IV – HBeAg-negative chronic hepatitis, V – HBsAg-negative phase [25]. During phase (V), HBsAg is not detected in the peripheral blood, but the viral genome remains in liver cells and can replicate at a low level or exist as integrated HBV DNA in human chromosomes. This condition is defined as occult hepatitis B [25, 26].
The EASL Guidelines define occult hepatitis B infection (OBI) based on the following parameters [24]: negative HBsAg in serum; presence of antibodies to the core antigen of hepatitis B virus (anti-HBc); normal alanine aminotransferase levels; presence or absence of HBV DNA in serum, but mandatory presence of cccDNA capable of replication in the liver of patients. In cases where HBsAg is present but not detectable using available commercial tests, for example, due to mutations in the S gene, chronic hepatitis B cannot be classified as the V form – OBI [27].
At the end of December 2024, the Ministry of Health of Russia approved the updated Clinical Guidelines for Chronic Viral Hepatitis B, developed by the V.I. Pokrovsky National Association of Specialists in Infectious Diseases and the Russian Scientific Liver Society The classification of chronic hepatitis B by phases of the infectious process coincides with the one provided above [24]. The fifth phase of the infection is designated as the «HBsAg-negative phase (OBI)»[2].
The aim of the review is to summarize the available data on OBI, draw attention to the significance of OBI and virus reactivation in diseases of the liver and other organs, identify problems requiring further study, and point out prospects for their solution.
The prevalence of occult hepatitis B
The assessment of the prevalence of OBI varies significantly in the works of different authors and, as noted, depends on the prevalence of the virus in different regions of the world. The determination of OBI is conducted in different population groups, including the general population, among practically healthy individuals, among patients with chronic hepatitis B and other liver diseases, as well as in high-risk groups for OBI reactivation and adverse disease outcomes. The Table presents data obtained from the study of two populations in different countries and regions of the world: the general population and the donor population, which is the most frequently and thoroughly examined population. The results of individual studies, as well as the data from the meta-analysis of the OBI, are presented.
Table. Prevalence of occult hepatitis B in the general population and in the blood donor population in different regions and countries of the world
Таблица. Распространенность скрытого гепатита В в общей популяции и в популяции доноров крови среди населения в разных регионах и странах мира
Region/Country Регион/Страна | Surveyed contingent Обследованный контингент | Prevalence of OBI1 (%) Распространенность СГВ1 (%) | HBV DNA test, detection limit Тест на ДНК ВГВ, предел определения | Reference Ссылка | |
China Китай | General population Общая популяция | 6/52 (11.5) | Nested PCR Гнездовая ПЦР | [28] | |
Blood donors Доноры крови | 31/44 256 (0.070) | Nested PCR, ≤ 6 IU/ml Гнездовая ПЦР, ≤ 6 МЕ/мл | [29] | ||
Western Europe and North America Западная Европа и Северная Америка | General population Общая популяция | 92/329 (28)2 | PCR, various tests and detection limits in different countries: 1.69–154 IU/ml ПЦР, разные тесты и пределы определения в разных странах: 1,69–154 МЕ/мл | [30] | |
34 countries4 34 страны4 | General population Общая популяция | 1159/3 667 171 (0.82) | [31] | ||
25 countries 5 25 стран5 | Donors3 Доноры3 | > 10 000 000 (0.05) | [32] | ||
Vietnam Вьетнам | General population Общая популяция | 7/58 (12) | Nested PCR, ≤ 10 IU/ml Гнездовая ПЦР, ≤ 10 МЕ/мл | [33] | |
Blood donors Доноры крови | 2/623 (0.3) | Nested PCR, 30–40 copies/ml Гнездовая ПЦР, 30–40 копий/мл | [34] | ||
Africa Африка | Gambia Гамбия | General population Общая популяция | 31/330 (9.4) | Nested PCR, < 5 IU/ml Гнездовая ПЦР, < 5 МЕ/мл | [35] |
Cameroon Камерун | Blood donors Доноры крови | 11/240 (4.5) | qPCR, 25 IU/mL ПЦРрв, 25 МЕ/мл | [36] | |
6 African countries6 6 стран Африки6 | General population Общая популяция | > 1 000 000 (13.3) (0–35.6) | PCR, different tests and detection limits in different countries ПЦР, разные тесты и пределы определения в разных странах | [31] | |
Various regions of Africa Разные регионы Африки | Blood donors3 Доноры крови3 | (3.18)7 | PCR, different tests and detection limits in different countries ПЦР, разные тесты и пределы определения в разных странах | [37] | |
Russia Россия | General population Общая популяция | 0/544 (0) | qPCR, 100 IU/mL ПЦРрв, 100 МЕ/мл | [38] | |
Blood donors Доноры крови | 76/2788 (2.85) | Nest PCR Гнездовой ПЦРрв | [39] | ||
Blood donors Доноры крови | 7/2492 (0.28) | PCR, 100 copies/ml ПЦР, 100 копий/мл | [40] | ||
Note. 1 – absence of HBsAg, presence of anti-HBc and HBV DNA in serum/plasma; 2 – liver biopsy samples; 3 – meta-analysis of data; 4 – countries of Europe, Africa, North and South America, Asia; 5 – countries of Europe, Asia, Africa, North and South America, Oceania; 6 – Botswana, Burkina Faso, Egypt, Nigeria, South Africa, Uganda; 7 – 86 publications present the results of a survey of 4074,603 blood donors, the number of donors with OBI was studied in 14 publications, the total number of donors with OBI is indicated only as a percentage. qPCR – quantitative real-time PCR.
Примечание. 1 – отсутствие HBsAg, присутствие анти-HBc и ДНК ВГВ в сыворотке/плазме крови; 2 – образцы биопсии печени; 3 – метаанализ данных; 4
The data presented in the table showed that the prevalence of OBI in the general population, when studying blood serum, varies significantly: from 0% (among 540 healthy residents of Russia in Moscow) and 0.82% (among more than 3 million examined people in 34 countries worldwide) to 11.5% in China, 12% in Vietnam, and 9.4–13.3% in African countries. These data confirm the correlation between the prevalence of OBI and the level of HBV endemicity. The highest prevalence of HBV is observed in the Asia-Pacific and African regions, where more than half of the world’s population resides. According to WHO data, in 2022, these regions accounted for at least 63% of new HBV infections worldwide and approximately 1.08 million deaths3. This is reflected in the high prevalence of OBI: for example, in China, according to R. Xia (2022), there are 21 million people living with OBI [41]. It should be noted in particular that when studying liver samples, OBI is detected significantly more often than in blood serum. Thus, as a result of studying liver samples in Western Europe and North America, OBI was detected in the general population in 28% of cases, whereas in the analysis of blood serum from 34 countries, it was detected 34 times less frequently – in 0.82% of cases (table). Principally similar data were obtained by Y.R. Im et al. (2022) as a result of a meta-analysis: the prevalence of OBI markers in 140,518,289 serum or plasma samples was on average 0.09%, while in 2,598 liver samples it was 34.8% [42]. According to the authors [43], data on the detection of HBV DNA in the liver are presented in only 10% of all published studies on OBI. It can be assumed that the prevalence of OBI is significantly broader than currently known based on the analysis of only serum or plasma.
A comparative analysis of the data in the table showed that among blood donors in almost all regions and countries of the world, the frequency of detecting OBI was significantly lower than in the general population (from 2–4 times to 100 times or more), however, even in this population, there was significant data variability. According to the meta-analysis by G.R. Takuissu et al. (2022), the prevalence of OBI among blood donors varied from 0.7% (in Europe) to 16.7% (in Southeast Asia with high endemicity of HBV) and overall was 6.2% [44]. Experts acknowledge that blood transfusions from donors with OBI pose a risk of transmitting HBV, with the residual risk of transmission estimated at 3–14% [45]. To prevent the transmission of the virus, it is recommended to screen blood donors using highly sensitive serological and molecular tests [32].
It should be noted that the currently available data do not allow for an assessment of the global prevalence of OBI in the general population, as the size of the surveyed groups is relatively small, especially for large countries. Moreover, the studies use tests with varying sensitivity, and standards have not yet been established, which complicates the comparative assessment of the obtained results.
The largest number of studies is dedicated to the analysis of high-risk populations for developing hepatitis B. These include: patients with chronic hepatitis B and other liver diseases; patients on hemodialysis; cancer patients; patients co-infected with HBV and other viruses, as well as individuals with weakened immune systems. The results of the analysis of OBI in high-risk groups are summarized in several studies from recent years. Analysis of OBI in Western countries with relatively low levels of chronic hepatitis B showed very high data variability: among patients with cryptogenic cirrhosis or progressive liver fibrosis, the prevalence of OBI ranged from 4 to 38%; among HIV-infected patients – from 0 to 45%; among patients on hemodialysis – from 0 to 54%; among patients co-infected with HCV – around 52%; and even among blood donors, who represent a low-risk group, the prevalence of OBI ranged from 0 to 22.7% [46].
An assessment of the prevalence of OBI in 34 countries and regions showed [31] that the prevalence of OBI significantly differs in high-risk groups compared to the general population. Thus, in the general population, the meta-analysis determined the prevalence of OBI at an average of 0.82%, whereas it was 13.99% among patients with liver diseases, 16.26% among people with HIV infection and 4.25% among patients on hemodialysis. Y.R. Im et al. (2022) compared the prevalence of OBI in countries with different endemicity of HBV. The meta-analysis showed that in countries with low endemicity, the prevalence of OBI in the blood donor population was on average 0.06%, while in countries with high endemicity, the prevalence amounted to 0.98% [42]. The assessment of the prevalence of OBI in high-risk groups conducted by these authors revealed higher values: in countries with low endemicity, it was 5.5%, while in countries with high endemicity, it was 12.0% [42].
A meta-analysis of OBI in high-risk groups in Egypt showed a high prevalence of OBI: while the prevalence ranged from 1.26% to 4.16% in the general population [47], it amounted to 17% in patients on hemodialysis, 41% in patients with multiple transfusions, 15% in patients with chronic hepatitis C, 31% in patients with liver cancer, and 13% in patients with liver cirrhosis [48]. Among HIV-infected patients in various regions of Africa, OBI was identified in an average of 11.2% of cases [49]. It should be noted that in Mexico, OBI was found among HIV-infected patients much more frequently – in 18/50 (36%) [50], than on average among blood donors – in 6.4% of cases [51].
In Russia, as well as in other countries, the prevalence of OBI was uneven among different population groups. For example, among 544 healthy residents of Moscow, no cases of OBI were identified (table), whereas among hematological patients, the same authors found OBI in 5 out of 129 (3.9%) patients [38]. Significantly more often – in 7 out of 35 (20%) cases, OBI was detected in patients with gastrointestinal diseases [52]. An assessment of the prevalence of OBI among blood donors by certain authors showed the presence of OBI markers in 76 out of 2788 donors (2.85%) [39]; whereas other authors found OBI among blood donors 10 times less frequently – in 7 out of 2492 (0.28%) cases [40]. At the same time, among individuals using psychoactive substances (PAS), these same authors found OBI almost 18 times more frequently – in 3/62 (4.94%) cases.
Important data were obtained based on 76 studies of OBI in cohorts of individuals vaccinated against hepatitis B. OBI was detected in 1–37% of fully vaccinated individuals, including children born to vaccinated mothers [53]. This means that, despite the significant success of the universal vaccination program against HBV, reactivation of the virus in OBI cannot be ruled out, and to eliminate the virus and OBI in the human population, more effective OBI vaccines need to be implemented in clinical practice.
Summarizing the data presented above, it can be noted that there is significant variability in the results regarding the prevalence of OBI. These discrepancies can be explained by a whole range of circumstances, such as:
- small sample sizes in many, if not most, studies; examination of populations from countries and geographical regions with varying endemicity of hepatitis B;
- analysis of heterogeneous populations differing by sex, age and risk of developing adverse outcomes of hepatitis B;
- examination of patients with various liver diseases and at different stages of the pathological process, including cryptogenic cirrhosis and progressive liver fibrosis;
- the presence of other diseases, including parenteral viral infections of HCV and HIV etiology;
- the use of methods and test systems with varying sensitivity and specificity for the detection of HBsAg and HBV DNA in the liver and/or serum, which does not exclude the possibility of obtaining false-positive and false-negative results;
- various types of biological material: serum/plasma, blood cells (PBMC), liver biopsies, paraffin-embedded liver sections;
- the use of an inconsistent (often insufficiently complete) set of markers in determining the OBI.
Clinical significance of occult hepatitis B
Opinions among researchers regarding the clinical significance of OBI are ambiguous. The absence of HBsAg in the serum/plasma of patients with chronic hepatitis B is often considered as the resolution of the disease, including after treatment [54–56].
At the same time, many studies provide data on the negative impact of OBI on the clinical condition of patients with chronic hepatitis B and the association of OBI with unfavorable outcomes of chronic hepatitis B [57, 58]. It is noted that people with OBI can infect others and that this form of infection may be associated with serious complications such as liver cirrhosis and hepatocellular carcinoma (HCC). [59].
Occult hepatitis B in liver fibrosis and cirrhosis
In patients with OBI, HBV replication is generally suppressed, and a low viral load is detected, usually less than 200 international units (IU) per 1 mL of plasma/serum [47]. However, the examination of patients with chronic hepatitis B showed that the presence of HBV DNA in the absence of HBsAg may be associated with a severe course of hepatitis B, the development of fibrosis, and liver cirrhosis. Thus, in a study of 83 patients with OBI, liver fibrosis was detected in 52 (62.6%) of them [60]. X. Tang et al. (2023) [61] examined 1772 patients with liver fibrosis and determined that 148 of them (8.4%) had OBI. Comparative analysis showed that in patients with OBI, the levels of all fibrosis markers (hyaluronic acid (HA), laminin, procollagen type III peptide (PCIII), collagen type IV (CIV), and the histological activity index (HAI)) were significantly higher than in 1624 patients with fibrosis but without OBI (p < 0.05). Moreover, HBV DNA was significantly more frequently detected in liver biopsy cells by in situ hybridization in patients with OBI than in patients without OBI (80.6% vs. 37.5%). The authors concluded that OBI is associated with a severe form of liver fibrosis.
Patient examinations show that in at least 10% of people, the etiology of chronic liver diseases remains undetermined [62]. In such cases, liver diseases, including cirrhosis, are referred to as cryptogenic [63]. Attempts to determine the presence of OBI in patients with cryptogenic chronic liver diseases have been described. In Iran, OBI was detected in 2/104 (1.9%) patients with cryptogenic chronic hepatitis [64]. It should be noted that a low-sensitivity variant of the polymerase chain reaction (PCR) method was used for the analysis of HBV DNA – 150 × 103 copies/mL. Other authors from Iran found OBI in 11 out of 29 (38%) patients with cryptogenic liver cirrhosis. OBI DNA was detected using real-time PCR and nested PCR methods, with DNA concentrations in patients with OBI ranging from 22 to 7138 copies/mL [65]. In another study from Iran, 7 out of 50 (14%) patients with cryptogenic liver cirrhosis had HBV DNA detected in their serum and significantly elevated transaminase levels. In the control group, none of the 80 healthy volunteers had HBV DNA detected in their serum. Nested PCR was used with a sensitivity for detecting HBV DNA in serum of 19 IU/mL [66]. One possible explanation for the significant variability of results from a single endemic country may be the different composition of patient groups, as well as the different sensitivity of methods for detecting viral DNA.
Analysis of the causes of liver cirrhosis in 111 patients [67] showed the presence of hepatitis B markers – HBsAg and anti-HBc – in the serum of 66 patients, while serological markers of viral hepatitis were not detected in 18 patients, who were classified as having cryptogenic cirrhosis. The study of HBV DNA showed its presence in the serum of 7/18 (38.9%) patients, which allowed for the diagnosis to be established in these patients. To determine the causes of liver cirrhosis in 68 patients who underwent liver transplantation, frozen liver samples were analyzed, and OBI was detected in 3 (4.4%) patients, while in 2 out of 3 patients with cirrhosis, the cause of the disease had not been previously established [68]. Later, the same authors studied liver biopsy samples from 104 HBsAg-negative patients with various liver diseases of unknown etiology and detected HBV DNA in 7 out of 104 patients (6.7%). The authors concluded that for diagnosing cryptogenic liver diseases, it is necessary to conduct tests that can detect low concentrations of viral DNA characteristic of occult hepatitis B [69].
Liver cirrhosis correlates with HBV infection in approximately 42% of cases [42] and is the most important factor contributing to the development of liver cancer [70]. According to data from large-scale retrospective studies, patients with cirrhosis, despite HBsAg clearance, have a 2–10 times higher risk of developing HCC compared to patients without cirrhosis [71–74]. This shows that liver cirrhosis associated with OBI can be a precancerous condition [75].
Occult hepatitis B and hepatocellular carcinoma
HCC was one of the six most common types of cancer and the third leading cause of cancer mortality worldwide in 2020 [76]. In China, primary liver cancer is the 5th most common type of cancer and ranks 2nd in terms of mortality [77].
As mentioned in the Introduction of the study, HBV DNA integration often occurs at an early stage of infection, but can also occur at later stages, including in patients with HBsAg clearance [11]. Integrated HBV sequences, found in the majority (70–90%) of HCC associated with chronic hepatitis B [78], can cause insertional mutagenesis and genomic rearrangements; however, the regulatory mechanisms of these processes are still insufficiently studied [79, 80].
Data on the development of HCC in patients with OBI are of interest. Chinese researchers noted spontaneous clearance of HBsAg in 55 out of 1355 patients with chronic hepatitis B. Over 23 months of observation, 18 out of 55 (32.7%) developed serious complications, including 11 (20%) with HCC. The authors concluded that the absence of HBsAg does not exclude the risk of developing HCC [81]. In another study, seroclearance of HBsAg was determined in 298 patients with chronic hepatitis B, and HBV DNA was detected in the liver of all of them. In the serum, HBV DNA was detected in 13.4% within 1 year, in 6.1% after 5–10 years, and in 3.7% of patients more than 10 years after the disappearance of HBsAg; 7 patients (2.4%) developed HCC. It was determined that the risk of developing liver fibrosis and HCC was higher in patients with OBI over the age of 50 (p = 0.004) [82]. T.C. Yip et al. (2017) [83] observed seroclearance of HBsAg in 4568 patients after treatment with nucleoside analogs or interferon. After 1–5 years, 54 patients (1.2%) were diagnosed with HCC. The study results showed that after the disappearance of HBsAg, the risk of developing HCC is higher in women over 50 years of age and in men of any age.
Long-term therapy that suppresses HBV DNA activity can reduce the incidence and mortality of HCC; however, complete cure of chronic hepatitis B is currently virtually impossible, as there are no reliable methods to eliminate integrated viral DNA from the human genome to date [84, 85]. In this regard, the term «functional cure» is used in the guidelines of the American Association for the Study of Liver Diseases (AASLD) and EASL, which is defined as the sustained absence of HBsAg and HBV DNA levels in serum below the quantitative detection limit 24 weeks after the start of treatment [86]. A term with a similar definition – «functional response» to treatment, is provided in the clinical guidelines approved in Russia in 20242.
At the same time, data have been published showing that after treating patients with chronic hepatitis B and suppressing viral activity, the risk of HCC remains. It should be noted that the frequency of developing HCC in patients after HBsAg seroclearance varies significantly in reports from different authors, ranging from 0 to 20% [85, 87, 88].
In many recent studies, it is indicated that patients with OBI have an increased risk of HCC [89, 90]. The results of the study by D.K.H. Wong et al. showed that the majority (69%) of HBsAg-negative patients with HCC had OBI, among which 29 patients were found to have cccDNA in liver cells and 43 patients had HBV DNA integrated into hepatocyte DNA near oncogenes [90]. In another study, 90 out of 251 (35.8%) patients with chronic hepatitis were diagnosed with HCC and focal liver lesions [91]. Importantly, among patients with cryptogenic HCC, 73% were found to have OBI, and HBV DNA was more frequently detected in tissues adjacent to the tumor [92].
It can be concluded that in the absence of HBsAg and the detection of HBV DNA, the risk of developing HCC remains, and this necessitates monitoring patients with OBI for early detection of the tumor. Moreover, researchers note that studying OBI in liver diseases is crucial for achieving the WHO goal of eradicating viral hepatitis by 2030 [56].
Data is accumulating on the role of OBI not only in liver tumors but also in malignant tumors of other organs [93]. In one study, in 4 out of 5 patients with pancreatic cancer, whose blood serum was negative for HBsAg but positive for anti-HBc, the expression of the HBV X gene was detected in the tumor tissue. In 3 patients, cccDNA of HBV was detected in pancreatic cells, and in one patient, integrated HBV DNA was found [94]. The authors believe that the basis of carcinogenesis could be fragments of integrated viral DNA and HBx expression.
They indicate a link between HBV and a high risk of developing B-cell lymphomas, especially in countries where HBV is endemic [95]. Examination of patients with HBV and B-cell lymphoma showed that chemotherapy and immunotherapy with rituximab cure 60–65% of patients; however, those who do not respond to therapy have an unfavorable prognosis [96, 97]. A meta-analysis of studies from different regions of the world showed that the likelihood of developing non-Hodgkin lymphomas in HBV-infected individuals is 2–3 times higher than in non-infected individuals [98, 99].
This means that patients with malignant lymphoproliferative diseases, including lymphomas, should be screened for the presence of HBV infection markers before starting therapy, and also monitored for HBV reactivation, which is often observed as a result of comprehensive lymphoma therapy.
Reactivation of hepatitis B virus in combined infections and during immunosuppressive therapy
Reactivation of HBV occurs in most cases in individuals with chronic hepatitis B or OBI when immunity is weakened, which is observed: 1) in the elderly (over 60 years old), more often in men, and with severe consequences of hepatitis (cirrhosis); 2) in patients co-infected with other pathogens; 3) in patients with oncological and other diseases receiving immunosuppressive therapy. Below are the studies that confirm these observations.
Co-infection with hepatitis B virus (HBV) and hepatitis C virus (HCV). According to the U.S. Food and Drug Administration (FDA), between 2013 and 2016, 29 cases of HBV reactivation were reported in patients with hepatitis C who were receiving direct-acting antiviral agents (DAA). One patient died, and another required a liver transplant [100]. Later, in patients with OBI infected with HCV, reactivation of HBV after DAA treatment was detected in an average of 3% of cases [101]. Similar data were obtained in another study [102]: three months after hepatitis C treatment with sofosbuvir and daclatasvir, HBV reactivation was observed in 4 out of 140 patients (2.8%). Significantly more often – in 81/111 (73%) patients with hepatitis C and HBV – HBV reactivation was recorded after treatment with ledipasvir and sofosbuvir. Clinical manifestations of hepatitis were observed in 10 out of 111 (9%) patients [103]. Reactivation of HBV was observed in a patient with OBI who was receiving ibrutinib [104]. After hepatitis C treatment, HBV reactivation occurred more frequently in patients with current HBV co-infection – in 10 out of 29 (34.5%) patients, but also, although significantly less frequently, in patients with OBI – in 3 out of 228 (1.3%) [105]. In contrast, S. Meschi et al. [106] reported that among 137 patients with HBV/HCV coinfection, HBV reactivation occurred in 10% of patients with active hepatitis B and in 90% of those with a past (occult) infection. The authors used highly sensitive tests and believe that for analyzing the risk of HBV reactivation, it is necessary to determine not only HBsAg but also HBV DNA. Q. Maqsood et al. (2023) disagree with this, showing that markers such as HBV DNA and pregenomic HBV RNA are not effective for predicting HBV reactivation, and that only HBsAg titer can be used as a biomarker for HBV reactivation [107]. All authors agree that the risk of HBV reactivation in co-infection with HCV is high, and this is explained by the fact that HCV inhibits HBV replication, whereas the suppression of HCV as a result of DAA treatment can lead to HBV reactivation [106, 108]. These assumptions are in line with current recommendations, which state that all patients with HCV who are planned to undergo DAA treatment should be screened for HBsAg and HBV DNA [109].
New coronavirus infection. COVID-19 has been recognized as another cause of HBV reactivation, as its treatment involves the administration of high doses of corticosteroids and/or certain immunosuppressive drugs. Thus, in the examination of patients with severe COVID-19 and markers of OBI who received the immunosuppressive drug tocilizumab, reactivation of HBV was detected in 2 out of 23 patients (9.7%). The authors concluded that in such cases, prophylaxis with entecavir should be conducted [110]. These results are consistent with data on HBV reactivation in 4/23 (17.4%) patients with COVID-19 who received immunosuppressants: in 1/8 (12.5%) HBsAg-positive patients and in 3/15 (20%) with OBI [111]. The authors recommend testing all patients with COVID-19 receiving immunosuppressive therapy for the 3 markers of HBV infection – HBsAg, anti-HBs, anti-HBc – and, if indicated, conducting prophylaxis for HBV reactivation.
HIV infection. Reactivation of HBV in HIV-infected individuals is facilitated by the immune suppression caused by HIV infection. A summary of the results from 50 studies showed that the global prevalence of HBV among children and adolescents was 7.5%, whereas among HIV-infected individuals it was three times higher at 24.2% [112]. Reactivation of HBV was observed in a large group of HIV-infected women before the initiation of antiretroviral therapy. In 8 out of 400 (2%) patients, HCV was detected. The results of the study showed that HIV-infected patients with OBI more often developed immunosuppression (CD4 cell count < 200 cells/mm3) and had higher levels of HIV RNA [113].
Oncological diseases. A high-risk group for HBV reactivation consists of cancer patients. According to available data, in cancer patients with chronic hepatitis B, the frequency of HBV reactivation due to antitumor therapy can range from 30% to 80%, depending on the drugs used, the chemotherapy regimen, and the patient’s serological status [114]. Reactivation of HBV can lead to severe hepatitis, liver failure, or death [115]. It was previously reported that the risk of reactivation of past hepatitis B in lymphoma patients undergoing chemotherapy is 11% [116]. A recently conducted meta-analysis, including 328 cancer patients with chronic hepatitis undergoing chemotherapy, showed that 3% of them (10 cases) experienced HBV reactivation. There are studies reporting that anti-HBV prophylaxis before the start of antitumor therapy can significantly reduce HBV reactivation in patients with oncological diseases [117]. The authors emphasized the importance of screening all patients for not only HBsAg but also anti-HBc before chemotherapy for tumors, and noted that the Centers for Disease Control and Prevention and AASLD also recommended testing for HBV markers in individuals receiving immunosuppressive therapy.
It has been established [118] that the risk of HBV reactivation depends on the specific immunosuppressive agent and whether the patient currently had chronic hepatitis B (HBsAg+) or OBI (HBsAg−, anti-HBc+, HBV DNA+). Thus, in patients with chronic hepatitis B, the highest risk of reactivation (> 10%) was associated with the use of anti-CD20 agents after hematopoietic stem cell transplantation (HSCT), whereas in patients with OBI, it was associated with the use of Janus kinase (JAK) inhibitors. Numerous immunosuppressive drugs are also known to pose a moderate (1–10%) and low (< 1%) risk of HBV reactivation in patients with tumors. It has been established that the rate of HBV reactivation in cancer patients with a history of chronic hepatitis B after chemotherapy is on average 25% (4–68%). In the majority (65%) of these patients, disease progression was observed, which required liver transplantation, otherwise leading to death [119]. Experts recommend that all patients with positive HBsAg undergo hepatitis B prophylaxis before starting immunosuppressive therapy. As for patients with OBI, the risk of reactivation varies significantly and depends on the use of different immunosuppressive drugs, thus it is recommended to prescribe specific hepatitis B prophylaxis in each individual case. It is noted that in patients without HBsAg but with anti-HBc, who were on immunosuppressive therapy without prophylaxis, HBV reactivation can reach more than 10% [120, 121].
Immune mechanisms play a key role in the reactivation of HBV. Disruptions in the host’s immune responses against infected cells, as well as disruptions in the signaling pathways of the interferon system, allow the virus to evade the host’s immune defense and contribute to reactivation. One of the priority areas in combating HBV reactivation is the selection of antiviral drugs in each specific case. It has been shown that the implementation of personalized medicine – treatment approaches tailored to the individual patient – can significantly influence the prevention of HBV reactivation [122]. The authors emphasize the importance of comprehensive treatment for HBV reactivation, combining immunomodulation methods with antiviral therapy.
Conclusion
OBI is attracting increasing attention from specialists of various profiles, as evidenced by the growing number of studies dedicated to this issue. The connection between OBI and such serious consequences of chronic hepatitis B as liver cirrhosis and HCC, as well as with malignant tumors of other organs, has been demonstrated. Analysis of OBI markers allows for the diagnosis in cases of cryptogenic liver diseases. It has been established that OBI is a risk factor for the reactivation of HBV, especially with a decrease in immunity. Reactivation of the virus leads to a more severe course of hepatitis and an unfavorable outcome of the disease. High-risk patients include those with chronic hepatitis B co-infected with other viruses: hepatitis C virus (HCV), SARS-CoV-2 and HIV. A high risk of HBV reactivation has been established in cancer patients with OBI undergoing chemotherapy. The possibility of HBV reactivation in patients after blood transfusions from OBI-positive donors, as well as the transmission of HBV to organ recipients during organ transplants from OBI-positive donors, has been demonstrated.
A series of measures must be taken to resolve the issue of OBI. These include large-scale screening of the general population, as well as high-risk groups for virus reactivation and the development of hepatitis B. Due to the low concentrations of HBV DNA in serum/plasma, as well as the inability to obtain liver samples in most cases, it is necessary to improve the diagnosis of OBI by increasing the sensitivity of the tests used in clinical practice. The prospects of using new HBV markers are being considered: HBV RNA and HBcrAg (Hepatitis B core-related antigen). HBcrAg is a new surrogate biomarker whose level correlates with the level of cccDNA of HBV and allows for the assessment of the presence and level of HBV DNA in the liver without the use of invasive procedures. The lack of therapeutics to target cccDNA currently does not allow for the removal of the virus from the body. To disrupt or suppress the activity of cccDNA, approaches based on small-molecule inhibitors and CRISPR/Cas genome editing technology are being developed. Addressing the issues of OBI will contribute to achieving the WHO goal of eliminating viral hepatitis by 2030.
1 WHO. Hepatitis B; 2024. Available at: https://who.int/news-room/fact-sheets/detail/hepatitis-b
2 The rubricator of clinical recommendations. Available at: https://cr.minzdrav.gov.ru
3 WHO. Global Hepatitis Report 2024. Action for access in low- and middle-income countries; 2024. Available at: https://who.int/publications/b/68511
About the authors
Alla A. Kushch
N.F. Gamaleya National Research Centre for Epidemiology and Microbiology, Ministry of Health of the Russian Federation
Author for correspondence.
Email: vitallku@mail.ru
ORCID iD: 0000-0002-3396-5533
D.Sci. (Biol.), Prof., Senior Researcher at the Laboratory of Cell Engineering
Russian Federation, 123098, MoscowReferences
- Ou T.Y., Huy L.D., Mayne J., Shih C.L., Mai Xuan H., Thi Hong Nguyen N., et al. Global mortality of chronic liver diseases attributable to Hepatitis B virus and Hepatitis C virus infections from 1990 to 2019 and projections to 2030. J. Infect. Public Health. 2024; 17(7): 102443. https://doi.org/10.1016/j.jiph.2024.04.027.
- Tsukuda S., Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020; 182: 104925. https://doi.org/10.1016/j.antiviral.2020.104925
- Mouzannar K., Schauer A., Liang T.J. The post-transcriptional regulatory element of hepatitis B virus: from discovery to therapy. Viruses. 2024; 16(4): 528. https://doi.org/10.3390/v16040528
- Panasiuk Ya.V., Vlasenko N.V., Churilova N.S., Klushkina V.V., Dubodelov D.V., Kudryavtseva E.N., et al. Modern views on the role of X gene of the hepatitis B virus (Hepadnaviridae: Orthohepadnavirus: Hepatitis B virus) in the pathogenesis of the infection it causes. Voprosy virusologii. 2022; 67(1): 7–17. https://doi.org/10.36233/0507-4088-84 https://elibrary.ru/tvufhi (in Russian)
- Li D., Hamadalnil Y., Tu T. Hepatitis B viral protein HBx: Roles in viral replication and hepatocarcinogenesis. Viruses. 2024; 16(9): 1361. https://doi.org/10.3390/v16091361.
- Asami J., Park J.H., Nomura Y., Kobayashi C., Mifune J., Ishimoto N., et al. Structural basis of hepatitis B virus receptor binding. Nat. Struct. Mol. Biol. 2024; 31(3): 447–54. https://doi.org/10.1038/s41594-023-01191-5
- Iwamoto M., Saso W., Sugiyama R., Ishii K., Ohki M., Nagamori S., et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl Acad. Sci. USA. 2019; 116(17): 8487–92. https://doi.org/10.1073/pnas.1811064116
- Gómez-Moreno A., Ploss A. Mechanisms of hepatitis B virus cccDNA and minichromosome formation and HBV gene transcription. Viruses. 2024; 16(4): 609. https://doi.org/10.3390/v16040609
- Gopalakrishna H., Ghany M.G. Perspective on emerging therapies to achieve functional cure of chronic hepatitis B. Curr. Hepatol. Rep. 2024; 23(2): 241–52. https://doi.org/10.1007/s11901-024-00652-9
- Sinha P., Thio C.L., Balagopal A. Intracellular host restriction of hepatitis B virus replication. Viruses. 2024; 16(5): 764. https://doi.org/10.3390/v16050764
- Yu X., Gong Q., Yu D., Chen Y., Jing Y., Zoulim F., et al. Spatial transcriptomics reveals a low extent of transcriptionally active hepatitis B virus integration in patients with HBsAg loss. Gut. 2024; 73(5): 797–809. https://doi.org/10.1136/gutjnl-2023-330577
- Zoulim F., Chen P.J., Dandri M., Kennedy P.T., Seeger C. Hepatitis B virus DNA integration: Implications for diagnostics, therapy, and outcome. J. Hepatol. 2024; 81(6): 1087–99. https://doi.org/10.1016/j.jhep.2024.06.037
- Yeh S.H., Li C.L., Lin Y.Y., Ho M.C., Wang Y.C., Tseng S.T., et al. Hepatitis B virus DNA integration drives carcinogenesis and provides a new biomarker for HBV-related HCC. Cell. Mol. Gastroenterol. Hepatol. 2023; 15(4): 921–9. https://doi.org/10.1016/j.jcmgh.2023.01.001
- Gu Z., Jiang Q., Abulaiti A., Chen X., Li M., Gao N., et al. Hepatitis B virus enhancer 1 activates preS1 and preS2 promoters of integrated HBV DNA impairing HBsAg secretion. JHEP Rep. 2024; 6(9): 101144. https://doi.org/10.1016/j.jhepr.2024.101144
- Maiorella R., Rodriguez V.A. Hepatitis B vaccine refusal in the newborn period. Pediatr. Ann. 2021; 50(8): e343–7. https://doi.org/10.3928/19382359-20210712-01
- Rajbhandari R., Chung R.T. Treatment of hepatitis B: A concise review. Clin. Transl. Gastroenterol. 2016; 7(9): e190. https://doi.org/10.1038/ctg.2016.46
- Michalak T.I., Pasquinelli C., Guilhot S., Chisari F.V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Invest. 1994; 93(1): 230–9. https://doi.org/10.1172/JCI116950
- Hirode G., Choi H.S.J., Chen C.H., Su T.H., Seto W.K., Van Hees S., et al. Off-therapy response after nucleos(t)ide analogue withdrawal in patients with chronic hepatitis B: An international, multicenter, multiethnic cohort (RETRACT-B Study). Gastroenterology. 2022; 162(3): 757–71.e4. https://doi.org/10.1053/j.gastro.2021.11.002.
- Di Dato F., Iorio R. Expanding indications for chronic hepatitis B treatment: Is it really desirable to treat everyone? World J. Gastroenterol. 2024; 30(17): 2294–7. https://doi.org/10.3748/wjg.v30.i17.2294
- Gerlich W.H., Bremer C., Saniewski M., Schüttler C.G., Wend U.C., Willems W.R., et al. Occult hepatitis B virus infection: detection and significance. Dig. Dis. 2010; 28(1): 116–25. https://doi.org/10.1159/000282074
- Kleinman S.H., Busch M.P. Assessing the impact of HBV NAT on window period reduction and residual risk. J. Clin. Virol. 2006; 36(Suppl. 1): S23–9. https://doi.org/10.1016/s1386-6532(06)80005-3
- Golubeva I.F., Shal’nova E.E., Bochkova G.B. Infection risks and ensuring the safety of donated blood and its components. Laboratornaya diagnostika infektsionnykh zabolevaniy. 2019; (1): 6–21. (in Russian)
- Heim K., Sagar, Sogukpinar Ö., Llewellyn-Lacey S., Price D.A., Emmerich F., et al. Attenuated effector T cells are linked to control of chronic HBV infection. Nat. Immunol. 2024; 25(9): 1650–62. https://doi.org/10.1038/s41590-024-01928-4
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017; 67(2): 370–98. https://doi.org/10.1016/j.jhep.2017.03.021
- Budzinska M.A., Shackel N.A., Urban S., Tu T. Cellular genomic sites of hepatitis B virus DNA integration. Genes (Basel). 2018; 9(7): 365. https://doi.org/10.3390/genes9070365
- Raimondo G., Locarnini S., Pollicino T., Levrero M., Zoulim F., Lok A.S., et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019; 71(2): 397–408. https://doi.org/10.1016/j.jhep.2019.03.034
- Wang C., Xue R., Wang X., Xiao L., Xian J. High-sensitivity HBV DNA test for the diagnosis of occult HBV infection: commonly used but not reliable. Front. Cell. Infect. Microbiol. 2023; 13: 1186877. https://doi.org/10.3389/fcimb.2023.1186877
- Fang Z.L., Zhuang H., Wang X.Y., Ge X.M., Harrison T.J. Hepatitis B virus genotypes, phylogeny and occult infection in a region with a high incidence of hepatocellular carcinoma in China. World J. Gastroenterol. 2004; 10(22): 3264–8. https://doi.org/10.3748/wjg.v10.i22.3264
- Mo Y., Jin F., Li D., Zou W., Zhong J., Tong Z., et al. Prevalence and molecular characteristics of occult hepatitis B virus infection among blood donors in Huzhou City, eastern China. Gene. 2024; 927: 148718. https://doi.org/10.1016/j.gene.2024.148718
- Pisaturo M., Onorato L., Russo A., Chiodini P., Coppola N. An estimation of the prevalence of occult HBV infection in Western Europe and in Northern America: A meta-analysis. J. Viral Hepat. 2020; 27(4): 415–27. https://doi.org/10.1111/jvh.13248
- Ji D.Z., Pang X.Y., Shen D.T., Liu S.N., Goyal H., Xu H.G. Global prevalence of occult hepatitis B: A systematic review and meta-analysis. J. Viral Hepat. 2022; 29(5): 317–29. https://doi.org/10.1111/jvh.13660
- Fu M.X., Faddy H.M., Candotti D., Groves J., Saa P., Styles C., et al. International review of blood donation screening for anti-HBc and occult hepatitis B virus infection. Transfusion. 2024; 64(11): 2144–56. https://doi.org/10.1111/trf.18018
- Serikova E.N., Semenov A.V., Ostankova Yu.V., Totolian A.A. A method for detecting hepatitis B virus in blood plasma at low viral load using real-time PCR. Klinicheskaya laboratornaya diagnostika. 2021; 65(1): 59–64. https://doi.org/10.18821/0869-2084-2021-66-1-59-64 https://elibrary.ru/fagbfm (in Russian)
- Tung T.T., Schmid J., Nghia V.X., Cao L.C., Linh L.T.K, Rungsung I., et al. Low risk of occult hepatitis B infection among Vietnamese blood donors. Pathogens. 2022; 11(12): 1524. https://doi.org/10.3390/pathogens11121524
- Ndow G., Cessay A., Cohen D., Shimakawa Y., Gore M.L., Tamba S., et al. Prevalence and clinical significance of occult hepatitis B infection in the Gambia, West Africa. J. Infect. Dis. 2022; 226(5): 862–70. https://doi.org/10.1093/infdis/jiab327
- Mbencho M.N., Hafza N., Cao L.C., Mingo V.N., Achidi E.A., Ghogomu S.M., et al. Incidence of Occult Hepatitis B Infection (OBI) and hepatitis B genotype characterization among blood donors in Cameroon. PLoS One. 2024; 19(10): e0312126. https://doi.org/10.1371/journal.pone.0312126.
- Simpore A., Bazie B.V.E.J.T., Yooda P.A., Zoure A.A., Sawadogo S., Sawadogo A.G., et al. Seroprevalence of viral hepatitis B and occult hepatitis B among blood donors in Africa: a systematic review and meta-analysis. Rev. Med. Virol. 2024; 34(6): e70006. https://doi.org/10.1002/rmv.70006
- Semenenko T.A., Yarosh L.V., Bazhenov A.I., Nikitina G.Yu., Kleimenov D.A., Elgort D.A., et al. Epidemiological assessment of the prevalence of “occult” forms and HBsAg mutants of hepatitis B virus in hematological patients. Epidemiologiya i vaktsinoprofilaktika. 2012; (6): 9–14. (in Russian)
- Ostankova Yu.V., Serikova E.N., Shirshova N.Yu., Kuseviczkaya M.B., Gorskaya O.A., Basina V.V., et al. Prevalence of latent form of chronic hepatitis B among blood donors in St. Petersburg. Infektsiya i immunitet. 2023; 13(6): 1129–40. https://doi.org/10.15789/2220-7619-POO-14480 https://elibrary.ru/xhpybw (in Russian)
- Ganina A.A., Kyuregyan K.K., Isaeva O.V., Dmitriev P.N., Mardanly S.G., Michailov M.I. The frequency of detection of “occult” hepatitis B among people with drug addiction and blood donors. Narkologiya. 2008; 7(9): 70–4. https://elibrary.ru/kaybtf (in Russian).
- Xia R., Peng J., He J., Jiang P., Yuan C., Liu X., et al. The serious challenge of occult hepatitis B virus infection-related hepatocellular carcinoma in China. Front. Microbiol. 2022; 13: 840825. https://doi.org/10.3389/fmicb.2022.840825
- Im Y.R., Jagdish R., Leith D., Kim J.U., Yoshida K., Majid A., et al. Prevalence of occult hepatitis B virus infection in adults: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022; 7(10): 932–42. https://doi.org/10.1016/S2468-1253(22)00201-1.
- Bucio-Ortiz L., Enriquez-Navarro K., Maldonado-Rodríguez A., Torres-Flores J.M., Cevallos A.M., Salcedo M., et al. Occult hepatitis B virus infection in hepatic diseases and its significance for the WHO’s elimination plan of viral hepatitis. Pathogens. 2024; 13(8): 662. https://doi.org/10.3390/pathogens13080662
- Takuissu G.R., Kenmoe S., Amougou Atsama M., Atenguena Okobalemba E., Mbaga D.S., Ebogo-Belobo J.T., et al. Global epidemiology of occult hepatitis B virus infections in blood donors, a systematic review and meta-analysis. PLoS One. 2022; 17(8): e0272920. https://doi.org/10.1371/journal.pone.0272920
- Candotti D., Boizeau L., Laperche S. Occult hepatitis B infection and transfusion-transmission risk. Transfus. Clin. Biol. 2017; 24(3): 189–95. https://doi.org/10.1016/j.tracli.2017.06.014
- Pisaturo M., Onorato L., Russo A., Coppola N. Prevalence of occult HBV infection in Western countries. J. Med. Virol. 2020; 92(12): 2917–29. https://doi.org/10.1002/jmv.25867
- Elbahrawy A., Alaboudy A., El Moghazy W., Elwassief A., Alashker A., Abdallah A.M. Occult hepatitis B virus infection in Egypt. World J. Hepatol. 2015; 7(12): 1671–8. https://doi.org/10.4254/wjh.v7.i12.1671
- Azzam A., Khaled H., El-Kayal E.S., Gad F.A., Omar S. Prevalence of occult hepatitis B virus infection in Egypt: a systematic review with meta-analysis. J. Egypt. Public Health Assoc. 2023; 98(1): 13. https://doi.org/10.1186/s42506-023-00138-4
- Kajogoo V.D., Swai S.S., Gurung S. Prevalence of occult hepatitis B among HIV-positive individuals in Africa: A systematic review and meta-analysis. SAGE Open Med. 2022; 10: 20503121211072748. https://doi.org/10.1177/20503121211072748
- Enriquez-Navarro K., Maldonado-Rodriguez A., Rojas-Montes O., Torres-Ibarra R., Bucio-Ortiz L., De la Cruz M.A., et al. Identification of mutations in the S gene of hepatitis B virus in HIV positive Mexican patients with occult hepatitis B virus infection. Ann. Hepatol. 2020; 19(5): 507–15. https://doi.org/10.1016/j.aohep.2020.06.002
- García-Montalvo B.M., Ventura-Zapata L.P. Molecular and serological characterization of occult hepatitis B infection in blood donors from Mexico. Ann. Hepatol. 2011; 10(2): 133–41.
- Morozov I.A., Il’chenko L.Yu., Gromova N.I., Fyodorov I.G., Gordeychuk I.V., Knyazhentseva A.K., et al. Problems of latent infection caused by the hepatitis B virus. Rossiyskiy zhurnal gastroenterologii, gepatologii, koloproktologii. 2012; 22(4): 58–65. https://elibrary.ru/piwdpl (in Russian)
- Delghandi S., Raoufinia R., Shahtahmasbi S., Meshkat Z., Gouklani H., Gholoobi A. An overview of occult hepatitis B infection (OBI) with emphasis on HBV vaccination. Heliyon. 2024; 10(17): e37097. https://doi.org/10.1016/j.heliyon.2024.e37097
- Yip T.C., Wong G.L., Wong V.W., Tse Y.K., Lui G.C., Lam K.L., et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J. Hepatol. 2017; 68(1): 63–72. https://doi.org/10.1016/j.jhep.2017.09.018
- Liu W.J., Wu W.J., Lin C.L., Liu C.J., Huang Y.W., Hu J.T., et al. Impact of age at HBsAg seroclearance on hepatic outcomes and life expectancy in men with chronic HBV infection based on multi-state modeling of the natural history. J. Gastroenterol. 2025; 60(1): 107–17. https://doi.org/10.1007/s00535-024-02162-3
- Leowattana W., Leowattana P., Leowattana T. Quantitative hepatitis B core antibody and quantitative hepatitis B surface antigen: Novel viral biomarkers for chronic hepatitis B management. World J. Hepatol. 2024; 16(4): 550–65. https://doi.org/10.4254/wjh.v16.i4.550
- El Jamaly H., Eslick G.D., Weltman M. Meta-analysis: hepatitis B reactivation in patients receiving biological therapy. Aliment. Pharmacol. Ther. 2022; 56(7): 1104–8. https://doi.org/10.1111/apt.17155
- Morrone A., Fiorilli V., Cinti L., Roberto P., Ferri A.L., Visentini M., et al. Surface antigen serocleared hepatitis B virus infection increases the risk of mixed cryoglobulinemia vasculitis in male patients with chronic hepatitis C. Front. Immunol. 2024; 15: 1411146. https://doi.org/10.3389/fimmu.2024.1411146
- Saravanan S., Shankar E.M., Vignesh R., Ganesh P.S., Sankar S., Velu V., et al. Occult hepatitis B virus infection and current perspectives on global WHO 2030 eradication. J. Viral Hepat. 2024; 31(7): 423–31. https://doi.org/10.1111/jvh.13928
- Peng J., Yao X., Yuan C., Liu X., Xia R., He J., et al. The investigation of hepatitis B vaccine immune responses in occult hepatitis B virus-infected patients. Front Immunol. 2022; 13: 903685. https://doi.org/10.3389/fimmu.2022.903685
- Tang X., Yang L., Zhang P., Wang C., Luo S., Liu B., et al. Occult hepatitis B virus infection and liver fibrosis in Chinese patients. J. Infect. Dis. 2023; 228(10): 1375–84. https://doi.org/10.1093/infdis/jiad140
- Yurlov K.I., Masalova O.V., Kisteneva L.B., Khlopova I.N., Samokhvalov E.I., Malinovskaya V.V. et al. Human herpesviruses increase the severity of hepatitis. Biology (Basel). 2021; 10(6): 483. https://doi.org/10.3390/biology10060483
- Berasain C., Betés M., Panizo A., Ruiz J., Herrero J.I., Civeira M.P., et al. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut. 2000; 47(3): 429–35. https://doi.org/10.1136/gut.47.3.429
- Kaviani M.J., Behbahani B., Mosallaii M.J., Sari-Aslani F., Taghavi S.A. Occult hepatitis B virus infection and cryptogenic chronic hepatitis in an area with intermediate prevalence of HBV infection. World J. Gastroenterol. 2006; 12(31): 5048–50. https://doi.org/10.3748/wjg.v12.i31.5048
- Anvari F.A., Alavian S.M., Norouzi M., Mahabadi M., Jazayeri S.M. Prevalence and molecular analysis of occult hepatitis B virus infection isolated in a sample of cryptogenic cirrhosis patients in Iran. Oman Med. J. 2014; 29(2): 92–6. https://doi.org/10.5001/omj.2014.23
- Hashemi S.J., Hajiani E., Masjedizadeh A., Makvandi M., Shayesteh A.A., Alavinejad S.P., et al. Occult hepatitis B infection in patients with cryptogenic liver cirrhosis in southwest of Iran. Jundishapur J. Microbiol. 2015; 8(3): e16873. https://doi.org/10.5812/jjm.16873
- Agarwal N., Naik S., Aggarwal R., Singh H., Somani S.K., Kini D., et al. Occult hepatitis B virus infection as a cause of cirrhosis of liver in a region with intermediate endemicity. Indian J. Gastroenterol. 2003; 22(4): 127–31.
- Ferrari T.C., Xavier M.A., Vidigal P.V., Amaral N.S., Diniz P.A., Resende A.P., et al. Occult hepatitis B virus infection in liver transplant patients in a Brazilian referral center. Braz. J. Med. Biol. Res. 2014; 47(11): 990–4. https://doi.org/10.1590/1414-431X20143782
- Faria A.C., Correa B.H.M., Faria L.C., Vidigal P.V.T., Xavier M.A.P., Ferrari T.C.A. Occult hepatitis B virus infection in patients with chronic liver disease of different etiology in a Brazilian referral center: comparison of two different hepatitis B virus deoxyribonucleic acid amplification protocols: a cross-sectional study. Sao Paulo Med. J. 2022; 141(3): e2022147. https://doi.org/10.1590/1516-3180.2022.0147.R1.12072022
- Daher D., Seif El Dahan K., Cano A., Gonzales M., Ransom C., Jaurez E., et al. Hepatocellular carcinoma surveillance patterns and outcomes in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2024; 22(2): 295–304.e2. https://doi.org/10.1016/j.cgh.2023.08.003
- Kim G.A., Lee H.C., Kim M.J., Ha Y., Park E.J., An J., et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J. Hepatol. 2015; 62(5): 1092–9. https://doi.org/10.1016/j.jhep.2014.11.031
- Yip T.C., Wong G.L., Chan H.L., Tse Y.K., Lam K.L., Lui G.C., et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J. Hepatol. 2019; 70(3): 361–70. https://doi.org/10.1016/j.jhep.2018.10.014
- Yang H., Bae S.H., Nam H., Lee H.L., Lee S.W., Yoo S.H., et al. A risk prediction model for hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J. Hepatol. 2022; 77(3): 632–41. https://doi.org/10.1016/j.jhep.2022.03.032
- Yip T.C., Wong V.W., Lai M.S., Lai J.C., Hui V.W., Liang L.Y., et al. Risk of hepatic decompensation but not hepatocellular carcinoma decreases over time in patients with hepatitis B surface antigen loss. J. Hepatol. 2023; 78(3): 524–33. https://doi.org/10.1016/j.jhep.2022.11.020
- Varghese N., Majeed A., Nyalakonda S., Boortalary T., Halegoua-DeMarzio D., Hann H.W. Review of related factors for persistent risk of hepatitis B virus-associated hepatocellular carcinoma. Cancers (Basel). 2024; 16(4): 777. https://doi.org/10.3390/cancers16040777
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71(3): 209–49. https://doi.org/10.3322/caac.21660
- Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019; 394(10204): 1145–58. https://doi.org/10.1016/S0140-6736(19)30427-1
- Péneau C., Imbeaud S., La Bella T., Hirsch T.Z., Caruso S., Calderaro J., et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut. 2022; 71(3): 616–26. https://doi.org/10.1136/gutjnl-2020-323153
- Dias J.D., Sarica N., Cournac A., Koszul R., Neuveut C. Crosstalk between hepatitis B virus and the 3d genome structure. Viruses. 2022; 14(2): 445. https://doi.org/10.3390/v14020445
- Zhang M., Chen H., Liu H., Tang H. The impact of integrated hepatitis B virus DNA on oncogenesis and antiviral therapy. Biomark. Res. 2024; 12(1): 84. https://doi.org/10.1186/s40364-024-00611-y
- Huo T.I., Wu J.C., Lee P.C., Chau G.Y., Lui W.Y., Tsay S.H., et al. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology. 1998; 28(1): 231–6. https://doi.org/10.1002/hep.510280130
- Yuen M.F., Wong D.K., Fung J., Ip P., But D., Hung I., et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008; 135(4): 1192–9. https://doi.org/10.1053/j.gastro.2008.07.008
- Yip T.C., Chan H.L., Wong V.W., Tse Y.K., Lam K.L., Wong G.L. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J. Hepatol. 2017; 67(5): 902–8. https://doi.org/10.1016/j.jhep.2017.06.019
- Ji M., Hu K. Recent advances in the study of hepatitis B virus covalently closed circular DNA. Virol. Sin. 2017; 32(6): 454–64. https://doi.org/10.1007/s12250-017-4009-4
- Kaur S.P., Talat A., Karimi-Sari H., Grees A., Chen H.W., Lau D.T.Y., et al. Hepatocellular carcinoma in hepatitis B virus-infected patients and the role of hepatitis B surface antigen (HBsAg). J Clin Med. 2022; 11(4): 1126. https://doi.org/10.3390/jcm11041126.
- Ghany M.G., Buti M., Lampertico P., Lee H.M.; 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference Faculty. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: Report from the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference. J. Hepatol. 2023; 79(5): 1254–69. https://doi.org/10.1016/j.jhep.2023.06.002
- Gounder P.P., Bulkow L.R., Snowball M., Negus S., Spradling P.R., Simons B.C., et al. Nested case-control study: hepatocellular carcinoma risk after hepatitis B surface antigen seroclearance. Aliment. Pharmacol. Ther. 2016; 43(11): 1197–207. https://doi.org/10.1111/apt.13621
- Kuang X.J., Jia R.R., Huo R.R., Yu J.J., Wang J.J., Xiang B.D., et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J. Viral Hepat. 2018; 25(9): 1026–37. https://doi.org/10.1111/jvh.12905
- Mak L.Y., Wong D.K., Pollicino T., Raimondo G., Hollinger F.B., Yuen M.F. Occult hepatitis B infection and hepatocellular carcinoma: Epidemiology, virology, hepatocarcinogenesis and clinical significance. J. Hepatol. 2020; 73(4): 952–64. https://doi.org/10.1016/j.jhep.2020.05.042
- Wong D.K., Cheng S.C.Y., Mak L.L., To E.W., Lo R.C., Cheung T.T., et al. Among patients with undetectable hepatitis B surface antigen and hepatocellular carcinoma, a high proportion has integration of HBV DNA into hepatocyte DNA and no cirrhosis. Clin. Gastroenterol. Hepatol. 2020; 18(2): 449–56. https://doi.org/10.1016/j.cgh.2019.06.029
- Huang Y., Li W., Hu H.T., Ruan S.M., Xian M.F., Xie X.Y., et al. Contrast-enhanced US diagnostic algorithm of hepatocellular carcinoma in patients with occult hepatitis B. Abdom. Radiol. (NY). 2022; 47(2): 608–17. https://doi.org/10.1007/s00261-021-03343-x
- Wong D.K., Huang F.Y., Lai C.L., Poon R.T., Seto W.K., Fung J., et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology. 2011; 54(3): 829–36. https://doi.org/10.1002/hep.24551
- Morozov S., Batskikh S. Commentary: Hepatitis B virus infection: an insight into the clinical connection and molecular interaction between hepatitis B virus and host extrahepatic cancer risk. Front Immunol. 2023; 14: 1200405. https://doi.org/10.3389/fimmu.2023.1200405
- Batskikh S., Morozov S., Dorofeev A., Borunova Z., Kostyushev D., Brezgin S., et al. Previous hepatitis B viral infection-an underestimated cause of pancreatic cancer. World J. Gastroenterol. 2022; 28(33): 4812–22. https://doi.org/10.3748/wjg.v28.i33.4812
- Rosenberg M., Poluch M., Thomas C., Sindaco P., Khoo A., Porcu P. Hepatitis B virus and B-cell lymphoma: evidence, unmet need, clinical impact, and opportunities. Front. Oncol. 2023; 13: 1275800. https://doi.org/10.3389/fonc.2023.1275800
- Dunleavy K. Double-hit lymphoma: optimizing therapy. Hematology Am. Soc. Hematol. Educ. Program. 2021; 2021(1): 157–63. https://doi.org/10.1182/hematology.2021000247
- Barraclough A., Hawkes E., Sehn L.H., Smith S.M. Diffuse large B-cell lymphoma. Hematol. Oncol. 2024; 42(6): e3202. https://doi.org/10.1002/hon.3202
- Li M., Gan Y., Fan C., Yuan H., Zhang X., Shen Y., et al. Hepatitis B virus and risk of non-Hodgkin lymphoma: an updated meta-analysis of 58 studies. J. Viral. Hepat. 2018; 25(8): 894–903. https://doi.org/10.1111/jvh.12892
- Spradling P.R., Xing J., Zhong Y., Rupp L.B., Moorman A.C., Lu M., et al. Incidence of malignancies among patients with chronic hepatitis B in US Health Care Organizations, 2006–2018. J. Infect. Dis. 2022; 226(5): 896–900. https://doi.org/10.1093/infdis/jiac011
- Bersoff-Matcha S.J., Cao K., Jason M., Ajao A., Jones S.C., Meyer T., et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann. Intern. Med. 2017; 166(11): 792–8. Doi: https://doi.org/10.7326/m17-0377
- Pisaturo M., Macera M., Alessio L., Calò F., Coppola N. Hepatitis B virus (HBV) reactivation following pharmacological eradication of hepatitis C virus (HCV). Viruses. 2019; 11(9): 850. https://doi.org/10.3390/v11090850
- Osman H.A., Ghweil A.A., Sabry A.M., Mahdy R.E., Khodeary A. Management of patients with hepatitis B virus reactivation post-DAA treatment of chronic hepatitis C virus infection In HCV-HBV coinfected patients with pretreatment HBeAg seroconversion and early degree of hepatic fibrosis. Infect. Drug Resist. 2019; 12: 3067–73. https://doi.org/10.2147/IDR.S215974
- Liu C.J., Sheen I.S., Chen C.Y., Chuang W.L., Wang H.Y., Tseng K.C., et al. Ledipasvir/Sofosbuvir for patients coinfected with chronic hepatitis C and hepatitis B in Taiwan: follow-up at 108 weeks posttreatment. Clin. Infect. Dis. 2022; 75(3): 453–9. https://doi.org/10.1093/cid/ciab971
- Lam L.K., Chan T.S.Y., Hwang Y.Y., Mak L.Y., Seto W.K., Kwong Y.L., et al. Hepatitis B virus reactivation in seronegative occult hepatitis B patient receiving ibrutinib therapy. Virol. J. 2023; 20(1): 168. https://doi.org/10.1186/s12985-023-02140-w
- Toka B., Köksal A.Ş., Dertli R., Şirin G., Fidan S., Ülger Y., et al. Hepatitis B reactivation in patients treated with direct-acting antivirals for hepatitis C. Dig. Dis. 2022; 40(5): 635–43. https://doi.org/10.1159/000521298
- Meschi S., Mizzoni K., Leoni B.D., Galli C., Garbuglia A.R., Belladonna S., et al. Occult HBV infection in patients infected by HIV or HCV: Comparison between HBV-DNA and two assays for HBsAg. Viruses. 2024; 16(3): 412. https://doi.org/10.3390/v16030412
- Maqsood Q., Sumrin A., Iqbal M., Younas S., Hussain N., Mahnoor M., et al. Hepatitis C virus/hepatitis B virus coinfection: Current prospectives. Antivir. Ther. 2023; 28(4): 13596535231189643. https://doi.org/10.1177/13596535231189643
- Colombatto P., Palmisano E., Ricco G., Cavallone D., Oliveri F., Coco B., et al. Different kinetics of HBV-DNA and HBsAg in HCV coinfected patients during DAAs therapy. J. Clin. Med. 2022; 11(5): 1406. https://doi.org/10.3390/jcm11051406
- Conners E.E., Panagiotakopoulos L., Hofmeister M.G., Spradling P.R., Hagan L.M., Harris A.M., et al. Screening and testing for hepatitis B virus infection: CDC recommendations – United States, 2023. MMWR Recomm. Rep. 2023; 72(1): 1–25. https://doi.org/10.15585/mmwr.rr7201a1
- Rodríguez-Tajes S., Miralpeix A., Costa J., López-Suñé E., Laguno M., Pocurull A., et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J. Viral Hepat. 2021; 28(1): 89–94. https://doi.org/10.1111/jvh.13410
- Mihai N., Olariu M.C., Ganea O.A., Adamescu A.I., Molagic V., Aramă Ș.S., et al. Risk of hepatitis B virus reactivation in COVID-19 patients receiving immunosuppressive treatment: a prospective study. J. Clin. Med. 2024; 13(20): 6032. https://doi.org/10.3390/jcm13206032
- Wu J., He J., Xu H. Global prevalence of occult HBV infection in children and adolescents: A systematic review and meta-analysis. Ann. Hepatol. 2024; 29(1): 101158. https://doi.org/10.1016/j.aohep.2023.101158
- Tsui J.I., French A.L., Seaberg E.C., Augenbraun M., Nowicki M., Peters M., et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin. Infect. Dis. 2007; 45(6): 736–40. https://doi.org/10.1086/520989
- Hwang J.P., Vierling J.M., Zelenetz A.D., Lackey S.C., Loomba R. Hepatitis B virus management to prevent reactivation after chemotherapy: a review. Support Care Cancer. 2012; 20(11): 2999–3008. https://doi.org/10.1007/s00520-012-1576-7
- Shouval D., Shibolet O. Immunosuppression and HBV reactivation. Semin. Liver Dis. 2013; 33(2): 167–77. https://doi.org/10.1055/s-0033-1345722
- Chen K.L., Chen J., Rao H.L., Guo Y., Huang H.Q., Zhang L., et al. Hepatitis B virus reactivation and hepatitis in diffuse large B-cell lymphoma patients with resolved hepatitis B receiving rituximab-containing chemotherapy: risk factors and survival. Chin. J. Cancer. 2015; 34(5): 225–34. https://doi.org/10.1186/s40880-015-0015-9
- Mezzacappa C., Lim J.K. Management of HBV reactivation: challenges and opportunities. Clin. Liver Dis. (Hoboken). 2024; 23(1): e0143. https://doi.org/10.1097/CLD.0000000000000143
- Anvari S., Tsoi K. Hepatitis B virus reactivation with immunosuppression: a hidden threat? J. Clin. Med. 2024; 13(2): 393. https://doi.org/10.3390/jcm13020393
- Zhu Y., Li H., Wang X., Zheng X., Huang Y., Chen J., et al. Hepatitis B virus reactivation increased the risk of developing hepatic failure and mortality in cirrhosis with acute exacerbation. Front. Microbiol. 2022; 13: 910549. https://doi.org/10.3389/fmicb.2022.910549
- Papatheodoridis G.V., Lekakis V., Voulgaris T., Lampertico P., Berg T., Chan H.L.Y., et al. Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: A systematic review, meta-analysis, and expert opinion. J. Hepatol. 2022; 77(6): 1670–89. https://doi.org/10.1016/j.jhep.2022.07.003
- Savaliya B.P., Shekouhi R., Mubarak F., Manaise H.K., Jimenez P.B., Kowkabany G., et al. Risk of hepatitis B virus reactivation in cancer patients undergoing treatment with tyrosine kinase-inhibitors. World J. Gastroenterol. 2024; 30(24): 3052–8. https://doi.org/10.3748/wjg.v30.i24.3052
- Ma H., Yan Q.Z., Ma J.R., Li D.F., Yang J.L. Overview of the immunological mechanisms in hepatitis B virus reactivation: Implications for disease progression and management strategies. World J. Gastroenterol. 2024; 30(10): 1295–312. https://doi.org/10.3748/wjg.v30.i10.1295
Supplementary files