Total antibodies and neutralizing ability of convalescent sera against three different strains of SARS-CoV-2
- Authors: Palyanova N.V.1, Adamenko L.S.1, Kurskaya O.G.1, Saroyan T.A.1, Solomatina M.V.1, Sobolev I.A.1, Shestopalov A.M.1
-
Affiliations:
- Federal Research Center for Fundamental and Translational Medicine
- Issue: Vol 70, No 1 (2025)
- Pages: 78-86
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16711
- DOI: https://doi.org/10.36233/0507-4088-290
- EDN: https://elibrary.ru/qcllgf
- ID: 16711
Cite item
Abstract
The aim of the study was to assess the level of humoral immunity to SARS-CoV-2 in COVID-19 convalescents.
Materials and methods. We used ELISA for antibody quantitation, microneutralization test using three SARS-CoV-2 strains for neutralizing activity measurement, Illumina MiSeq platform for NGS sequencing and NextClade resource for phylogenetic analysis.
Results. The mean concentration of antibody in convalescents was 133.42 ± 7.2 BAU/mL and the value depended on neither the gender and age of the patients, nor on the time elapsed since COVID-19 infection. The studied SARS-CoV-2 strains were sequenced and deposited in the international GISAID database. According to genetic analysis: EPI_ISL_19424272 phylogenetically belongs to the B1.1 clade, EPI_ISL_19424271 – to B.1.1.397 clade, EPI_ISL_19424270 – to Delta B.1.617.2.122. There were no significant differences in the neutralizing ability of convalescent sera (those who had been ill 2–3 months before the study) for the first two variants of SARS-CoV-2 and it was significantly reduced for the Delta variant, which appeared in the Novosibirsk region later.
Conclusions. The neutralizing activity of convalescent sera was the highest against those variants of the virus that the patient had recovered from, while was reduced or absent against the new variant. The antibody developed to the original variants of the SARS-CoV-2 may not be effective enough against newly emerging strains due to the emergence of mutations in the virus that allow it to evade previously developed humoral immune response.
Keywords
Full Text
Introduction
In December 2019, an outbreak of a new coronavirus infection (COVID-19) began in China, which was recognized as a pandemic by the World Health Organization (WHO) in March 2020. In Russia, the incidence began to increase from March 2020, but the first wave was small compared to subsequent waves. By the end of 2020, 27,885 people in the Novosibirsk Region (population 2.8 million) had contracted the disease, according to official data1. Infection caused by SARS-CoV-2 can be latent (asymptomatic) [1] or cause a wide range of COVID-19 symptoms, from fever, asthenia or myalgia to pneumonia, with acute respiratory distress syndrome developing in the most severe cases [2]. Diagnosis of COVID-19 is based on detection of SARS-CoV-2 RNA using real-time polymerase chain reaction (qPCR). At the beginning of the pandemic in the Novosibirsk region, all patients with symptoms of acute respiratory infections, travelers, and persons at risk were tested [3], but the asymptomatic infections remained hidden both to the patients and to official statistics. One way to establish the true number of new coronavirus infection convalescents is to detect antibodies to SARS-CoV-2 by enzyme-linked immunosorbent assay (ELISA). By early 2021, measurement of specific antibody levels had become particularly relevant for the purpose of making vaccination decisions and monitoring vaccine efficacy. Vaccination at that time had just begun, and by the end of December 2020, only 1,280 residents of the region had been vaccinated2.
Initially, the level of protective antibodies was measured using different test systems from different manufacturers and with different levels of reliability. Later, quantitative and qualitative indicators were recalculated in international BAU (binding antibody units) according to WHO standards (WHO, NIBSC code 20/136). In the present study, both qualitative antibody test and quantitative test calculated in BAU/mL were used.
The presence of antibodies to SARS-CoV-2 is not in itself a reason to consider a patient or a group of persons protected from re-infection [4]. It is necessary to ascertain the neutralizing ability of these antibodies and, consequently, the level of protection against infection. Furthermore, the level of neutralization may be different for different variants of the virus.
Materials and methods
Sample characteristics
259 people participated in the study. The subjects were recruited randomly in the Federal Research Center for Fundamental and Translational Medicine (FRC FTM) and the A.V. Nikolaev Institute of Inorganic Chemistry (Siberian branch of the Russian Academy of Sciences). All study participants were over 18 years of age and signed informed consents to participate in the study. The design of the study was approved by the Biomedical Ethics Committee of the FRC FTM (protocol #8 of 11.03.2020). A total of 77 people agreed to complete the participant questionnaire. The questionnaire included a question about age and date of detection of SARS-CoV-2 RNA in swabs. By the time the study started, 63 respondents indicated on the questionnaire that they had already had COVID-19 at various times and 14 respondents did not provide this information. By the end of the study, none of the participants had been immunized or reported recurrent disease, but it cannot be stated that there were no recurrent asymptomatic cases in the group, as regular PCR testing for SARS-CoV-2 RNA was not performed. The age of the reporting respondents ranged from 18 years to 81 years with a median age of 55 years. The study population included 40% males and 60% females.
Due to the fact that not all study participants had their blood drawn the same number of times, and the time from undergoing COVID-19 to the start of the study differed, multiple groups were formed:
- the group of individuals who received a qualitative SARS-CoV-2 antibody assay (259 participants);
- the group of individuals who were tested for antibodies to SARS-CoV-2 in quantitative assay (94 participants);
- groups of individuals (7 groups) who underwent COVID-19 at 1, 2, 3, 4, 5, 6, and 7–9 months prior to the study (6, 23, 37, 38, 34, 17, 8 participants respectively);
- the dynamic antibody monitoring group: COVID-19 convalescents 3 months prior to the study who underwent blood sampling once a month for 4 consecutive months (8 participants);
- the group of respondents who indicated on the questionnaire that they had not had COVID-19 (14 participants);
- the neutralization group: individuals whose serum was tested not only for antibody concentration but also for neutralizing activity against SARS-CoV-2 (30 participants).
Analysis of serum antibodies to SARS-CoV-2
Blood sampling was performed in the procedure room of the FRC FTM clinic once a month. Qualitative analysis of antibodies to SARS-CoV-2 was performed monthly from November 2020 to November 2021 using SARS-CoV-2-IgG-ELISA-BEST reagent kits (Vector-Best, Russia). Each participant could donate blood one or more times. A total of 596 samples from 259 people were analyzed during this period.
For serum samples collected in January, February, March and April 2021 and preliminarily characterized as seropositive to SARS-CoV-2 by the qualitative total antibody test, total serum IgM and IgG to SARS-CoV-2 were quantified by ELISA using the SARS-CoV-2-AT total-ELISA-BEST reagent kits (Vector-Best, Russia): a total of 209 samples from 94 individuals.
Despite the emergence of an international standard, different manufacturers of test systems specify different threshold values for determining a positive, negative or doubtful test result. Thus, in [5] samples with a level of less than 25.6 BAU/mL were classified as negative, from 25.7 to 35.1 were considered borderline, with a level of more than 35.2 BAU/mL were considered positive, while in the Vector-Best test-system the manufacturer recommends to be guided by the following table.
Gradation for Vector-Best test system (according to the manufacturer’s data):
- 0 to 10 BAU/mL – negative, no antibodies present;
- 11–79 BAU/mL – viral neutralizing effect is low (decision to vaccinate);
- 80–149.9 BAU/ml – viral neutralizing effect only in 50% of cases (control in dynamics);
- more than 150 BAU/ml – virus neutralizing activity is strongly expressed in 100% of cases (sufficient level for protection, vaccination is not required);
- 500 and above – maximum antibody level developed (no vaccination required)
In the present study, the gradation of the Vector-Best kit manufacturer was used as the guide.
Production and characterization of SARS-CoV-2 strains for neutralization
3 different strains of SARS-CoV-2 were obtained (isolated) for neutralization by sera of the convalescents (Table 1). The choice of variants corresponded to the 3 first waves of the pandemic in the Novosibirsk region. The complete genomes of these SARS-CoV-2 strains were sequenced and their affiliation to phylogenetic clades was determined using NextClade3. Sequencing was performed using Illumina MiSeq platform and corresponding reagent kits (Illumina, USA). Prior to sequencing, reverse transcription and amplification of genomes were performed for subsequent sequencing. When preparing genomic libraries for SARS-CoV-2 sequencing, the ARTIC v3 primer set was used4. Full genome nucleotide sequences were assembled using bowtie software. SARS-CoV-2 sequences were deposited in the international GISAID database with the names (identifiers) EPI_ISL_19424270, EPI_ISL_19424271, EPI_ISL_19424272.
Table 1. SARS-CoV-2 strains used to test the neutralizing ability of sera
Таблица 1. Штаммы SARS-CoV-2, использованные для проверки нейтрализующей способности сывороток
Name Название | EPI_ISL_19424272 | EPI_ISL_19424271 | EPI_ISL_19424270 |
Clade Клада | B.1.1 | B.1.1.397 | B.1.617.2.122 |
Reference variant Референсный вариант | Wuhan Ухань | Wuhan Ухань | Delta Дельта |
Circulated in the Siberian Federal District Циркулировал в СФО | From the beginning of the pandemic until May 2021 С начала пандемии до мая 2021 г. | January 2021 – May 2021 Январь 2021 г. – май 2021 г. | June 2021 – January 2022 Июнь 2021 г. – январь 2022 г. |
Conventional designation in the article Условное обозначение в статье | Wuhan 1 Ухань 1 | Wuhan 2 Ухань 2 | Delta Дельта |
In our previous study, we determined which SARS-CoV-2 variants were prevalent in the Siberian Federal District (SFD) during different periods of the pandemic [6], which allowed us to determine the periods of circulation in the Novosibirsk region of variants genetically similar to the strains obtained in the present study (Table 1).
Since the virus variants belonging to the Delta clade appeared in the Novosibirsk region only in the summer of 2021, there were no patient exposure to them at the time of sera collection.
Neutralizing ability of sera against three SARS-CoV-2 variants
Twenty-nine patients with antibodies to SARS-CoV-2 and one participant with no disease and no antibodies were randomly selected for neutralizing capacity analysis. In all patients, serum obtained in January, February, or March was analyzed.
Neutralization reaction (NR) studies of sera were performed by micromethod in Vero cell culture grown on 96-well plates from TPP (Switzerland). The neutralization reaction was performed with a constant drug dose of 100 TCID50/mL for each strain. The patients’ sera were preheated in a water bath at 56 °C for 30 min, then 8 consecutive twofold dilutions were prepared, starting with undiluted serum. The sera were diluted with PBS (phosphate-buffered saline). A mixture of serum dilutions and working virus dilution in equal volumes was prepared. The mixture was incubated for 1 h at room temperature, then added to the wells of a 96-well plate with a Vero cell culture monolayer and incubated for 4 days at 37 °C, 5% CO2. The results of titration were taken into account visually by microscopic examination of the cell monolayer for the presence of cytopathic effect (CPE) on the 4th day after infection. The titer of serum was considered to be the reverse value of its last dilution, in which no signs of CPE were recorded. The following controls were placed in the NR: cell control (CC) – wells not infected with virus; serum negative control (C−) – serum in dilution 1/10; virus control (VC) – wells infected with virus in twofold dilution; control of virus working concentration – two consecutive 10-fold dilutions of working virus concentration (vc/10, vc/100) were prepared. The values of the controls were counted as follows: CC – intact cell monolayer, C−, VC and vc/10 – complete degeneration of the cell monolayer, vc/100 ‒ ½ of the infected wells had signs of CPE.
Statistical analysis
Statistical analysis was performed using Statistica 10.0 software. Reliability of differences between groups was assessed using the χ2 criterion and Student’s t-criterion. Confidence intervals for relative values were calculated using the EPITOOLS online calculator5.
Results
Qualitative analysis for antibodies to SARS-CoV-2
For November and December 2020, 69 samples were tested, of which 32 were positive (33.3%; CI 95%: 24.7–43.2%). For 10 months of 2021, 537 serum samples were tested, of which 434 were positive (80.8%; CI 95%: 77.3–83.9%). During the entire follow-up period for 259 study participants, 195 had antibodies to SARS-CoV-2 (75.3%; CI 95%: 69.7–80.2%).
Quantitative analysis for antibodies to SARS-CoV-2
During 4 months (January, February, March and April 2021), quantitative analysis of antibodies to SARS-CoV-2 was performed for sera that preliminarily showed the presence of antibodies in qualitative analysis (total of 209 samples from 94 individuals). Among all blood samples tested, the minimum amount of total antibodies to SARS-CoV-2 was 14 BAU/mL, the maximum was 190 BAU/mL, the mean was 133.42 ± 7.2 BAU/mL, and the median was 157.5 BAU/mL. Because a different amount of time elapsed from the time of illness to the start of the study for each patient, participants with similar time from illness were combined into 7 groups: 1, 2, 3, 4, 5, 6, 7–9 months from illness. The number of patients in each group was: 6, 23, 37, 38, 34, 17, 8 patients respectively (Fig. 1).
Fig. 1. Dynamics of the number of antibodies.
Average number of total antibodies 1, 2, 3, 4, 5, 6, 7–9 months after COVID-19, quantitative method, BAU/мл (N = 6, 23, 37, 38, 34, 17, 8).
Рис. 1. Динамика количества антител.
Среднее количество суммарных антител через 1, 2, 3, 4, 5, 6, 7–9 мес после перенесенного COVID-19, количественный метод, BAU/мл (N = 6, 23, 37, 38, 34, 17, 8).
The amount of antibodies did not depend on the sex and age of the patients, as well as on the time elapsed after COVID-19 (Fig. 1). The spread of values in group 1 was quite large, and the number of participants was small; it was not possible to obtain the level of reliability of differences between this group and the others. On average, the antibody level persisted for at least 6 months.
Study participants never infected by COVID-19
The control group was to consist of study participants who did not have COVID-19. When antibody levels were measured in this group, it was found that only one person out of 14 who reported not having COVID-19 actually had no antibodies to SARS-CoV-2. The others were positive in both qualitative and quantitative assays according to the test kit manufacturer’s table (greater than 11 BAU/mL). These individuals may have carried the disease asymptomatically. Interestingly, despite the asymptomatic course, we cannot claim that this cohort had a less robust immune response than symptomatic overexposure. The variation in antibody levels in this group was quite large (Fig. 2). Some participants in this group donated blood more than once, which allowed calculation of the mean antibody count over the 4 months of follow-up. For the others, the result of a single measurement is shown.
Fig. 2. The level of total IgM and IgG antibodies to SARS-CoV-2 in 14 study participants who did not have COVID-19 (according to the questionnaire), BAU/mL.
Mean values and measurement errors were calculated when possible (more than three antibody concentration measurements). Participant No. 1 had a negative result, the rest of the study participants had a positive result.
Рис. 2. Уровень суммарный IgM и IgG к SARS-CoV-2 у 14 участников исследования, не болевших COVID-19 (по данным анкетирования), BAU/мл.
При возможности (более 3 измерений уровня антител) были рассчитаны средние значения и погрешности измерений. Участник № 1 имел отрицательный результат, у остальных участников исследования ‒ положительный результат.
Dynamic monitoring of antibody levels for 4 months
It was possible to trace the dynamics of antibody levels over 4 months in 8 participants. For a correct comparison, the dynamics were observed in the largest group ‒ those who had the disease 3 months before the start of the study and who donated blood every month for 4 months. Three scenarios were observed in this group: a gradual drop in antibody levels, an increase in antibody levels, and a wave-like behavior of antibody levels. The difference between the minimum and maximum antibody levels averaged 28 ± 7.7 BAU/mL (Fig. 3). The difference in the scenarios is explained by the different situation in which the study participants may have been in. Thus, a drop in antibody levels is a natural process, although not very noticeable over such a short period of time. When re-infection occurs, antibody levels rise, even if the infection is asymptomatic.
Fig. 3. Change in the concentration of antibodies over 4 months.
Dynamic changes in antibody levels within 4 months in the group that had COVID-19 3 months before the start of the study, BAU/mL.
Рис. 3. Изменение количества антител в течение 4 мес.
Динамическое наблюдение за уровнем антител в течение 4 мес в группе лиц, переболевших COVID-19 за 3 мес до начала исследования, BAU/мл.
Analysis of the dynamics of antibody levels for participants who underwent blood sampling at least 2 times revealed a pattern: antibody levels in one person, although they could fluctuate, remained relatively stable (standard deviation 16.6 ± 3.5) throughout the study. Apparently, the antibody level is individual for each person and varies within small limits in the absence of additional influences (vaccination, disease).
Neutralizing activity of antibodies
Three virus strains were selected for neutralization and tested against sera collected in January, February and March 2021. When comparing the time of circulation in SFD [6] and the time of serum collection, it was found that the samples collected in early 2021 were from individuals who may have had contact with the first two virus variants but not with the third.
All 30 sera were tested against the 3 strains of SARS-CoV-2. For the participant who did not have antibodies to SARS-CoV-2, no neutralizing ability was also detected in the serum for any strain (Table 2).
Table 2. Neutralizing ability of sera and the concentration of antibodies to SARS-CoV-2 in 30 study participants
Таблица 2. Нейтрализующая способность сывороток и количество антител к SARS-CoV-2 у 30 участников исследования
№ | Months from illness Месяцев от болезни | Age Возраст | January 2021 Январь | February Февраль 2021 | March Март 2021 | April Апрель 2021 | Wuhan 1 Ухань 1 | Wuhan 2 Ухань 2 | Delta Дельта |
1 | 0 | 37 | 164,3 | 164,3 | 185,9 | 157,5 | 1 : 32 | 1 : 16 | 1 : 16 |
2 | 1 | 49 | 160,6 | 181,9 | 181,9 | 161 | 1 : 2 | 1 : 64 | 0 |
3 | 1 | 58 | ‒ | ‒ | 185,9 | 157,5 | 1 : 4 | 1 : 16 | 1 : 2 |
4 | 2 | 29 | 25,6 | 18,3 | ‒ | 21 | 1 : 8 | 1 : 4 | 1 : 8 |
5 | 2 | 41 | 171,6 | 167,9 | 190 | 164,5 | 1 : 4 | 1 : 32 | 0 |
6 | 2 | 25 | ‒ | 24,3 | 24,25 | ‒ | 1 : 4 | 1 : 8 | 0 |
7 | 2 | 80 | 167,9 | 185,9 | 190 | 161 | 1 : 64 | 1 : 32 | 0 |
8 | 2 | 47 | 43,8 | 36 | 32,3 | 21 | 1 : 4 | 1 : 4 | 1 : 2 |
9 | 2 | 68 | ‒ | 181,9 | 177,8 | 154 | 1 : 8 | 1 : 32 | 0 |
10 | 2 | 54 | 25,6 | ‒ | 20,2 | 21 | 1 : 4 | 1 : 8 | 0 |
11 | 2 | 64 | 171,6 | 185,9 | 185,9 | 157,5 | 1 : 128 | 1 : 128 | 1 : 64 |
12 | 3 | 64 | 175,2 | 185,9 | 185,9 | ‒ | 1 : 64 | 1 : 128 | 1 : 64 |
13 | 3 | 57 | 146 | 137,4 | 137,4 | 125 | 1 : 32 | 1 : 8 | 1 : 4 |
14 | 3 | 67 | 157 | 157 | 165,7 | 148,2 | 1 : 64 | 1 : 8 | 0 |
15 | 3 | 32 | 105,9 | 93 | 93 | ‒ | 1 : 32 | 0 | 0 |
16 | 3 | 49 | 69,4 | 55 | 44,5 | 35 | 1 : 32 | 1 : 8 | 0 |
17 | 3 | 35 | 157 | 157 | 170 | ‒ | 1 : 32 | 1 : 8 | 1 : 4 |
18 | 3 | 64 | 146 | 113,2 | 93 | 108,5 | 1 : 8 | 1 : 16 | 0 |
19 | 3 | 78 | 164,3 | 165,7 | 157,6 | 154 | 1 : 32 | 1 : 1,1 | 0 |
20 | 3 | 71 | 171,6 | 167,9 | 173,8 | 150,5 | 1 : 16 | 1 : 32 | 0 |
21 | 3 | 50 | ‒ | ‒ | 84,9 | ‒ | 1 : 16 | 1 : 8 | 1 : 8 |
22 | 3 | 59 | ‒ | 167,9 | 181,9 | 157,5 | ‒ | 1 : 32 | 0 |
23 | 3 | 63 | 167,9 | 173,8 | 157,6 | ‒ | 1 : 8 | 1 : 16 | 1 : 4 |
24 | 3 | 62 | 171,6 | 181,9 | 190 | ‒ | 1 : 64 | 1 : 128 | 1 : 64 |
25 | 3 | 66 | 164,3 | 160,6 | 177,8 | 154 | 1 : 16 | 1 : 32 | 1 : 16 |
26 | 3 | 65 | 167,9 | 160,6 | ‒ | 157,5 | 1 : 32 | 0 | 1 : 16 |
27 | 3 | 66 | 160,6 | 164,4 | 173,8 | 150,5 | 1 : 8 | 1 : 32 | 0 |
28 | Hadn’t COVID-19 Не болел | 36 | 0,0 | 4,0 | 4,0 | 0,0 | 0 | 0 | 0 |
29 | No data Нет данных | 57 | ‒ | ‒ | 121,25 | ‒ | 1 : 2 | 1 : 2 | 0 |
30 | No data Нет данных | 31 | 105,9 | 84,9 | 72,8 | 73,5 | 1 : 16 | 1 : 4 | 1 : 8 |
Note. «–» – no data; «0» – no neutralization.
Примечание. «–» – отсутствуют данные; «0» – отсутствует нейтрализация.
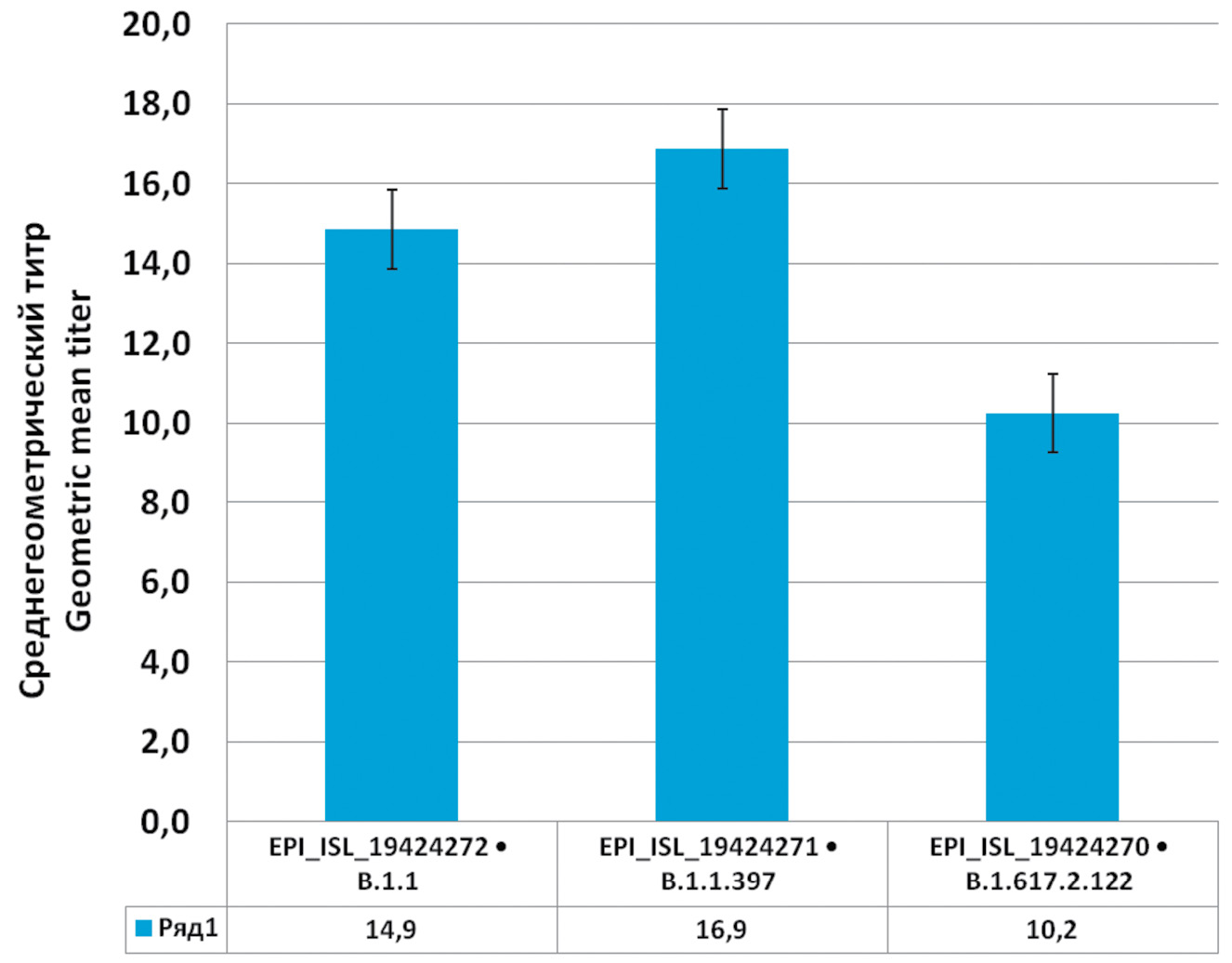
Against EPI_ISL_19424272 strain (Wuhan 1), one serum out of 29 seropositive sera did not show neutralization, against EPI_ISL_19424271 (Wuhan 2), 3 sera did not show neutralization, and against EPI_ISL_19424270 (Delta), 50% of the sera did not show neutralization. A geometric mean titer was calculated for the rest (Fig. 4).
Fig. 4. Neutralizing ability of convalescent serum against three strains of SARS-CoV-2: EPI_ISL_19424272 (Wuhan 1), EPI_ISL_19424271 (Wuhan 2) и EPI_ISL_19424270 (Delta).
Рис. 4. Нейтрализующая способность сывороток реконвалесцентов против 3 штаммов SARS-CoV-2: EPI_ISL_19424272 (Ухань 1), EPI_ISL_19424271 (Ухань 2) и EPI_ISL_19424270 (Дельта).
As shown above, the neutralizing ability of the sera of the convalescent patients has the highest activity against those variants of the virus that the patient has had, while against the new variant it is reduced or absent at all. It was also found that the level of neutralization does not depend on the concentration of antibodies and on whether the person has had the disease with or without symptoms. The group of patients whose sera showed no neutralization did not differ from the rest of the study participants. The level of antibodies to SARS-CoV-2, the severity of the disease course, and the time since illness did not affect the neutralization rate. Thus, the concentration of antibodies cannot serve as a reliable criterion of a person’s protection against the disease, since these antibodies could have been developed against an earlier variant of the virus.
Discussion
The mechanisms of immune response to SARS-CoV-2 are not yet fully understood. It is unclear whether or not persistent immunity develops in convalescents. The decision to vaccinate should be based not only on the epidemiologic situation but also on the immunity status of the vaccinated patient [7]. In the present study, we investigated the antibody component of the humoral immunity of the convalescents. The obtained results are consistent with the data of [5], where the average antibody level was 129.67 BAU/mL at the 3rd month after SARS-CoV-2 infection. We also looked at the dynamics of antibody counts at 4 months after COVID-19 3 months ago and found that although antibody levels varied significantly between patients, the changes for each patient did not vary as much. A large number of patients infected with SARS-CoV-2 were asymptomatic [1, 8], which does not allow us to sufficiently assess the incidence and potential for spread of this infection. We found no correlation between the asymptomatic course of the disease and antibody levels. Works on the neutralizing ability of sera are quite rare, because it is necessary to use live virus. Most studies present data on the formation of vaccine-induced immunity [9]. In our case, all study participants were not vaccinated, and the virus variants were selected so that it was possible to compare the neutralization of the variant circulating at the time of serum collection and the Delta variant, which was not encountered by the study participants. Since no correlation between antibody levels and neutralization titer was found, it is not possible to speak of protective humoral immunity based on antibody concentration alone.
Conclusion
With the ever-changing variants of SARS-CoV-2, information on the number of cases and the antibody levels of the patients may not be sufficient to make a vaccination decision, as new variants of the virus may effectively avoid previously developed immunity. Furthermore, immunity developed against early vaccine strains may not be sufficient to protect against subsequent SARS-CoV-2 variants, as is already the case with influenza virus.
1 Yandex DataLens. Coronavirus: dashboard. Available at: https://datalens.yandex/7o7is1q6ikh23?tab=X1&state=512ef5d71481 (in Russian)
2 Government of the Novosibirsk region. Vaccination against coronavirus continues in the Novosibirsk region. Available at: https://www.nso.ru/news/44654 (in Russian)
3 Nextclade. Available at: https://clades.nextstrain.org/
4 hCoV-2019/nCoV-2019 Version 3 Amplicon Set. Available at: https://artic.network/resources/ncov/ncov-amplicon-v3.pdf
5 Epitools – Calculate confidence limits for a sample proportion. Available at: https://epitools.ausvet.com.au/ciproportion
About the authors
Natalia V. Palyanova
Federal Research Center for Fundamental and Translational Medicine
Author for correspondence.
Email: natalia.palyanova@gmail.com
ORCID iD: 0000-0002-1783-5798
SPIN-code: 4975-4485
Scopus Author ID: 55983936800
ResearcherId: KAM-6786-2024
junior researcher
Russian Federation, 630117, NovosibirskLubov S. Adamenko
Federal Research Center for Fundamental and Translational Medicine
Email: aminisib@yandex.ru
ORCID iD: 0000-0001-6412-3622
junior researcher
Russian Federation, 630117, NovosibirskOlga G. Kurskaya
Federal Research Center for Fundamental and Translational Medicine
Email: kurskaya_og@mail.ru
ORCID iD: 0000-0002-1931-2026
Ph.D., Head of the Laboratory of Respiratory Viral Infections, Senior Researcher
Russian Federation, 630117, NovosibirskTereza A. Saroyan
Federal Research Center for Fundamental and Translational Medicine
Email: 111.st.13@rambler.ru
ORCID iD: 0000-0001-8071-5425
junior researcher
Russian Federation, 630117, NovosibirskMariya V. Solomatina
Federal Research Center for Fundamental and Translational Medicine
Email: solomatina.mariyav@yandex.com
ORCID iD: 0000-0003-0736-0271
Ph.D., senior researcher
Russian Federation, 630117, NovosibirskIvan A. Sobolev
Federal Research Center for Fundamental and Translational Medicine
Email: sobolev.riov@yandex.ru
ORCID iD: 0000-0002-4561-6517
Ph.D., Senior Researcher, Head of the Laboratory of Genomics and Virus Evolution
Russian Federation, 630117, NovosibirskAlexander M. Shestopalov
Federal Research Center for Fundamental and Translational Medicine
Email: shestopalov2@mail.ru
ORCID iD: 0000-0002-9734-0620
Ph.D., professor, Dr. of Sc., Honored Scientist of the Russian Federation, Director Research Institute of Virology FRC FTM
Russian Federation, 630117, NovosibirskReferences
- Balakhonov S.V., Dubrovina V.I., Chesnokova M.V., Voitkova V.V., Pyatidesyatnikova A.B., Bryukhova D.D., et al. Studying humoral immune response at mild and asymptomatic COVID-19 forms. Acta Biomedica Scientifica. 2020; 5(5): 26–30. https://doi.org/10.29413/ABS.2020-5.5.3 https://elibrary.ru/mvlnmh (in Russian)
- Machhi J., Herskovitz J., Senan A.M., Dutta D., Nath B., Oleynikov M.D., et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020; 15(3): 359–86. https://doi.org/10.1007/s11481-020-09944-5
- Palyanova N., Sobolev I., Alekseev A., Glushenko A., Kazachkova E., Markhaev A., et al. Genomic and epidemiological features of COVID-19 in the Novosibirsk region during the beginning of the pandemic. Viruses. 2022; 14(9): 2036. https://doi.org/10.3390/v14092036
- Novikova E.A., Petrova A.G., Moskaleva E.V., Vanyarkina A.S., Rychkova L.V. Retrospective of international serological studies on the formation and dynamics of the humoral immune response to SARS-CoV-2: from 2020 to 2021. Acta Biomedica Scientifica. 2021; 6(2): 47–57. https://doi.org/10.29413/ABS.2021-6.2.5 https://elibrary.ru/sbnnav (in Russian)
- Oktay Gültekin E., Gültekin O., Coskun A., Aksak T. Antibody response three months after SARS-CoV-2 infection. J. Med. Virol. 2022; 94(10): 4712–8. https://doi.org/10.1002/jmv.27909
- Palyanova N.V., Sobolev I.A., Palyanov A.Y., Kurskaya O.G., Komissarov A.B., Danilenko D.M. et al. The development of the SARS-CoV-2 epidemic in different regions of Siberia in the 2020–2022 Period. Viruses. 2023; 15(10): 2014. https://doi.org/10.3390/v15102014
- Tukhvatulin A.I., Dolzhikova I.V., Shcheblyakov D.V., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health Eur. 2021; 11: 100241. https://doi.org/10.1016/j.lanepe.2021.100241
- Evseeva G.P., Lazareva M.A., Vlasova M.A., Nagovitsyna E.В., Suprun S.V., Telepneva R.S. et al. Assessment of the level of immune layer to SARS-CoV-2 in children under conditions of novel coronavirus infection COVID-19. Byulleten’ fiziologii i patologii dykhaniya. 2023; (88): 59–68. https://doi.org/10.36604/1998-5029-2023-88-59-68 https://elibrary.ru/lhyxot (in Russian)
- Wang K., Long Q.X., Deng H.J., Hu J., Gao Q.Z., Zhang G.J., et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin. Infect. Dis. 2021; 73(3): e531–9. https://doi.org/10.1093/cid/ciaa1143
Supplementary files