Pre-clinical safety studies of intranasal virus-like particles based vaccine for prevention of COVID-19
- Authors: Chernoryzh Y.Y.1, Kondratieva V.M.1, Malkova А.P.2, Savochkina T.E.1, Eliseeva O.V.1, Latyshev O.E.1, Yakunin D.Y.1, Zaykova O.N.1, Sludnyakova E.S.1, Grebennikova T.V.1
-
Affiliations:
- N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
- Institute for Biomedical Research and Technology (IMBIIT)
- Issue: Vol 70, No 1 (2025)
- Pages: 35-46
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16701
- DOI: https://doi.org/10.36233/0507-4088-278
- EDN: https://elibrary.ru/fzgyxe
- ID: 16701
Cite item
Abstract
Introduction. The large-scale and prolonged pandemic of the novel coronavirus disease (COVID-19) has demonstrated the need for effective vaccination. Along with immunogenicity, safety is a critical issue for vaccines, as public trust can contribute to the success or failure of immunization programs. In preclinical studies, we assessed the safety of an intranasal Virus-like particle (VLP)-based vaccine in mice and rats.
The aim of the study is to conduct preclinical acute and subchronic toxicity studies assessing local tolerability of an intranasal VLP vaccine against COVID-19 in accordance with good laboratory practice.
Materials and methods. Study was performed on adult outbreed mice (30 males, 30 females) and rats (45 males, 45 females). Physiological, morphometric and histological parameters, as well as general and biochemical blood tests and urine analysis were assessed.
Results. No deaths or intoxication were recorded in the acute toxicity study on mice, all parameters were within the physiological norm. In the subchronic toxicity study on rats, no changes in the general condition, behavior, or death of animals were noted. The structure of internal organs, blood and urine tests, hemostasis did not differ significantly between the groups. No local irritant effect was detected at the injection site during visual assessment, cytological and histological analysis.
Conclusion. The VLP vaccine is safe, as evidenced by the results of preclinical studies, does not negatively affect the function of various organs, the level of cellular and biochemical biomarkers in the blood and urine of mice and rats. Visual assessment, cytology and histology of the vaccine injection site did not reveal any local irritant effect.
Keywords
Full Text
Introduction
The pandemic of a new coronavirus infection (COVID-19) has necessitated the World Health Organization (WHO) to declare a public health emergency, which has not been lifted for more than three years. Despite the success of global measures to control the spread of the COVID-19 pandemic, coronavirus infection can still pose a threat. This is due to the constant emergence of new variants of the SARS-CoV-2 virus, which arise from the accumulation of mutations in the natural replication process of the virus. SARS-CoV-2 virus is characterized by evolution at an approximate rate of 0.0011 substitutions per site per year, which corresponds to almost one substitution approximately every 11 days. At the end of 2020, WHO proposed a classification of new SARS-CoV-2 strains: 1) variants of interest (VOIs) – strains in which mutations resulted in a potential increase in disease severity due to reduced susceptibility to treatment and reduced neutralization by antibodies and/or transmissibility due to changes in virus binding to receptors; 2) variants of concern (VOCs) – strains with high transmissibility, higher disease severity and poor response to treatment and vaccines due to lack of neutralization by antibodies produced [1]. Studies aimed at finding an effective specific treatment for coronavirus infection have not yielded results; antiviral drugs targeting a wide range of viruses, including coronaviruses, and immunomodulators are used in treatment. Therefore, vaccination is currently the only means of prevention of the spread of coronavirus infection.
The urgent necessity for vaccination to combat the COVID-19 pandemic has led to a variety of COVID-19 vaccine platforms in use, including whole-virus inactivated vaccines, protein subunit vaccines, vector vaccines, DNA and/or mRNA-based vaccines, each with its own advantages. The main disadvantage is that most vaccines are based on generating immunity only to the surface spike S glycoprotein of SARS-CoV-2. Persistent and rapid mutations can lead to loss of epitopes in the S-protein in new strains and hence a predicted decrease in neutralization by vaccine-induced antibodies. Therefore, approaches to vaccine development and optimization must necessarily be based on predicting the impact of new VOCs to ensure that human immune responses are not evaded or suppressed by a constantly evolving virus.
VLP-based vaccine technology is a modern and promising approach to the development of immunogenic, effective and safe vaccines. VLPs are self-assembled structural viral proteins that do not contain the viral genome. VLP vaccines have a significant advantage over subunit vaccines, they present protein epitopes as native virus, allowing for a significantly enhanced immune response. Seven VLP-based vaccines against COVID-19 are undergoing clinical trials: LYB001 (Yantai Patronus Biotech Co Ltd), Covifenz (Medicago), NVX-CoV2373 (Novavax), VBI-2902a (VBI Vaccines Inc. ), ABNCoV2 (Radboud University), RBD SARS-CoV-2 HBsAg VLP vaccine (Serum Institute of India + Accelagen Pty + SpyBiotech), SARS-CoV-2 VLP Vaccine (The Scientific and Technological Research Council of Turkey), and nineteen are in preclinical studies1. Most vaccines, including approved and preclinical VLP vaccines, are characterized by parenteral administration (intramuscular or subcutaneous), which requires aseptic technique and specially trained personnel. To increase immunogenicity, they are used together with adjuvants of different nature, and the above vaccines are no exception. Intranasal immunization has demonstrated a promising ability to stimulate mucosal secretory immunity along with the formation of humoral and cellular immune response, and has a number of additional advantages, the main one being ease of administration and dosing compared to injectable forms. Protection of the respiratory tract, the entry gate for SARS-CoV-2 virus, by activation of local mucosal immunity is one of the main advantages of the intranasal route of vaccine administration. Compared to injectable forms, intranasal vaccine is convenient for dosing, nasal vaccine immunizations do not require specially trained personnel, thus eliminating the problem regarding shortage of specialists, which is important in the event of an epidemic and/or pandemic. Furthermore, the non-invasive method of vaccination is more attractive to people, especially those with trypanophobia, which significantly increases the number of patients who are willing to be immunized.
The quadrivalent intranasal VLP vaccine against COVID-19 is a mixture of 4 SARS-CoV-2 structural proteins (S, M, N, E) synthesized in a baculovirus expression system, purified and homogenized. VLPs mimic the SARS-CoV-2 virion, while containing no nucleic acids and therefore do not replicate in the body. The major immunogenic protein is the S-protein of SARS-CoV-2, and VLPs containing S-protein with consensus mutations of clades 19A, Alpha, Delta and Omicron are used in the vaccine formulation. Thus, after immunization, antibodies to these clade strains will be induced in the body [2, 3].
Materials and methods
Vaccine. Composition: Recombinant VLP complex containing S-protein on the surface, clades 19A, Alpha, Delta, Omicron SARS-CoV-2 synthesized in baculovirus expression system – 80–160 mcg; potassium dihydrophosphate – 0.63 mg; sodium hydrophosphate – 0.65 mg; sodium chloride – 3.84 mg; potassium chloride – 0.09 mg; calcium chloride – 0.02 mg; tris(hydroxymethyl)aminomethane-HCl – 0.03 mg; thiomersal – 4.00 µg; water for injection – up to 0.5 ml.
Animal models. Sexually mature outbred mice weighing 25–35 g (30 females (♀) and 30 males (♂)) and sexually mature outbred rats weighing 300-400 g (45♀ and 45♂) were used. Mice were housed 10 individuals each and rats 5 individuals each in polycarbonate cages with adaptations for food, water and steel label holders. The following groups were formed: “saline solution” – animals injected with saline solution (NaCl 0.9%); “squalene-based oil-in-water adjuvant” – group of animals injected intranasally with adjuvant; “VLP” – group of mice injected intranasally with 200 μg of vaccine antigen at a dose of; “VLP 80 μg” – group of rats that were intranasally injected with 80 μg antigen vaccine per dose; “VLP 160 μg” – group of rats that were intranasally injected with 160 μg antigen vaccine per dose. All animal manipulations were performed in accordance with the rules of the European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes ETS No. 123 (Strasbourg, 1986) and Directive 2010/63/EU of the European Parliament and of the Council of the European Union on the protection of animals used for scientific purposes. The design and concept of the study were developed taking into account the requirements of the Ministry of Health of the Russian Federation and the concept of humane use of animals in experiments – “The Three R’s”23 [4–14].
Selection of drug doses and route of administration. Mice were intranasally administered fractionally (25 μl at 1.5 h intervals) with a vaccine concentration of 200 μg antigen per dose for the acute toxicity study; this dose is more than 2000 times the human dose. Rats in the subchronic toxicity study of the VLP vaccine were administered intranasally one inoculum (80 µg antigen per dose) and double the human dose (160 µg antigen per dose). Similar to mice, due to the lack of possibility of a single intranasal administration of the tested doses, the administration was carried out fractionally (3 h interval).
Animal model observation. In the acute toxicity study on mice, the animals were monitored for two weeks, and on the day of vaccination, they were monitored hourly. In the chronic toxicity study on rats, 10 animals were removed from each group 24 hours after the last vaccine administration, and the remaining rats were monitored for two weeks. Mice and rats were euthanized with complete pre-treatment examination in the same manner as rats removed from the study groups 24 h after the last vaccine administration. Toxic effects were evaluated by criteria such as: animal deaths (timing and number if any); respiratory monitoring (dyspnea and/or tachypnea, cyanosis, rhinorrhea); motor monitoring (hyper/hypokinesia, hypersomnia, ataxia, loss of sensation, catalepsy, prostration, tremor, fascial convulsions); reflexes; ocular signs; hypersalivation; polyuria; cardiovascular monitoring (brady-, tachycardia, arrhythmia); gastrointestinal monitoring (dyspepsia, vomiting, stool changes); piloleiomyoma; alopecia; analgesia; muscle tone; feed and water consumption; zoosocial behavior.
Body mass registration. Weighing was carried out on ASOM PC-100W-5 scales (ASOM, South Korea).
Temperature measurement. Thermometry was performed rectally with an electronic medical thermometer B.Well WT-03 (B.Well, UK).
Water and feed consumption. Water consumption was determined by the difference in the volume of water in drinking bottles before and after 24 hours. Water consumption was determined by the difference in the volume of water in drinking bottles before placement in the cage and after 24 hours. Similarly, feed intake was determined by the difference in weight before distribution and after 24 hours.
Behavioral reactions in the “Open Field” test. The observation of animal behavior was carried out for 10 min in a special installation, which was a round square area with a diameter of 90 cm and a wall height of 50 cm, illuminated by a 40 W red lamp, which was placed at a distance of 2 m. Registration of the studied parameters was carried out visually. The time for adaptation, horizontal and vertical activity by the number of crossed squares horizontally and vertically, respectively, the number of acts of defecation and urination, as well as grooming (washing) were taken into account.
Cardiovascular activity readings such as heart rate and electrocardiographic activity were recorded using a Poly-Spectr-8/V computer electrocardiograph specialized for veterinary medicine (Neurosoft LLC, Russia).
Blood analysis. General blood analysis was performed on a Mindray BC-2800-vet hematological analyzer (Mindray, China) in accordance with the manufacturer’s instructions. For this purpose, blood was taken from the tail vein into tubes with a Univet EDTA anticoagulant. Such indicators as: number of red and white blood cells (erythrocytes and leukocytes-monocytes, lymphocytes and granulocytes) were evaluated. The following indices were determined in blood hemostasis tests: blood clotting time, activated partial thromboplastin time (APTT) and fibrinogen. blood was also collected from the tail vein, but in tubes with 3.8% sodium citrate solution. The analysis was performed on the analyzer of hemostasis parameters PGA-02-P (LLC EMKO, Russia) in accordance with the manufacturer’s instructions and reagent kits of Renam company.
Blood biochemical analysis was performed on a Stat Fax 4500+ photometer (Awareness Technology Inc., USA) using standard reagent kits from Unimed and Olvex Diagnostikum in accordance with the manufacturer’s instructions. Blood was collected in tubes with pellets for serum separation. The following parameters were determined: total protein (biuret method), aspartate and alanine aminotransferase and alkaline phosphatase (kinetic method), creatinine (kinetic method based on the Jaffe reaction), urea (UV kinetic method), albumin and sodium (colorimetric method), potassium (turbidimetric method without deproteinization), total bilirubin (by diazotization of bilirubin with diazosulfanilic acid), glucose (glucose oxidase method), cholesterol and triglycerides (enzymatic method).
Urine was analyzed using DecaFan LAURA strips on the LAURA Smart rapid urine analyzer (Erba Lachema s.r.o., Czech Republic). The specific gravity of urine, pH level and the content of erythrocytes, leukocytes, protein, glucose, ketones, bilirubin, urobilinogen and nitrite were determined.
Terminal procedures. Necropsy was performed immediately after the animals were sacrificed according to the complete pathologoanatomical scheme. When studying acute toxicity, euthanasia was performed by inhalation of carbon dioxide (CO2), and when studying subchronic toxicity, it was performed by decapitation with preliminary anesthesia.
Macroscopic examination. The following organs were visually examined: brain, heart, aorta, larynx, trachea, lungs, submandibular salivary gland, thyroid, thymus, lymph nodes, esophagus, stomach, pancreas, small and large intestines, liver and gallbladder, spleen, kidneys, adrenal glands, bladder, testes/ovaries, uterus and vagina.
Microscopic examination was performed using a Leica DM1000 light microscope (Leica Microsystems CSC GmbH, Germany) to analyze the cytohistoarchitectonics of the injection site (nasal passages).
Morphometric analysis. The organs were weighed on a DL-63 scale (DEMCOM, Russia). Paired organs were weighed together. The relative mass of organs (brain, thymus, heart, lungs, kidneys, adrenal glands, liver, spleen, testes/ovaries) was determined by the ratio of the mass of the studied organ to the animal body weight.
Histological examination. Slices (3-4 nm) of the studied organ and tissue samples were prepared on a microtome RMD-4000 (MT Point, Russia) after preliminary fixation in 15% formalin solution, as well as dehydration, degreasing and paraffinization on a histoprocessor TLP-144 (MT Point, Russia). After fixation of the obtained sections on a slide, deparaffinization in xylene and alcohol battery was performed for further staining with hematoxylin and eosin. Then the stained samples were examined on a Leica DM1000 light microscope (Leica Microsystems CSC GmbH, Germany).
Statistical analysis. The comparison criterion was chosen based on the parameters of normality of sample distribution. Reliability of the differences in the obtained values of the measured indicators between the control and experimental groups was performed using parametric and nonparametric methods. (Student’s t-test and Mann–Whitney U-test, respectively). A difference was considered significant at p < 0.05.
The authors confirm compliance with institutional and national standards for the use of laboratory animals in accordance with the Consensus author guidelines for animal use (IAVES 23.07.2010). The study protocol was approved by the Bioethical Commission of the Institute for Biomedical Research and Technologies (Protocol No. 4/2022 of 16.05.2022).
Results
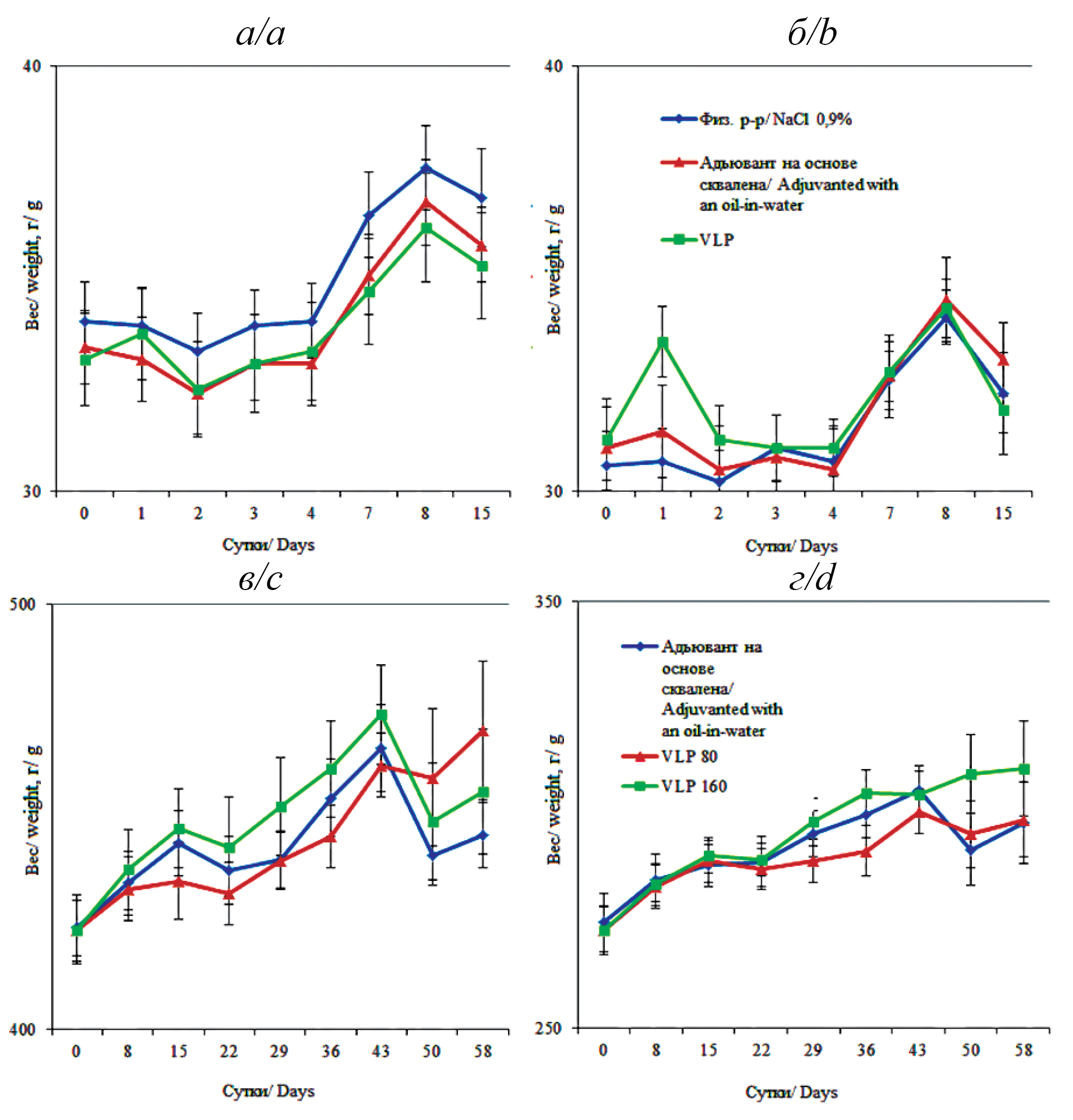
Acute toxicity study on mice showed that throughout the study the animals did not show clinical picture of intoxication, no deaths were registered. During immunization the animals behaved calmly, sneezing or ingestion of the drug was not observed. During the last injections of both the vaccine and the carrier, redness and swelling of the nasal speculum were observed in the mice, which passed within a day. After vaccine administration, as well as during the rest of the observation period, visual inspection of all animals of the studied groups showed no deviations from the norm and no differences between the groups. The mice looked to be in good health. Zoosocial behavior of mice of control and experimental groups did not differ. Appearance, coat condition, muscle tone and respiration were without peculiarities. Urinary frequency, urine color, gastrointestinal parameters, and reflexes corresponded to the physiological norm [15, 16]. No significant difference in feed and water consumption was recorded regardless of sex or study group, weight gain was not statistically significantly different between control (“saline solution”) and experimental groups (“Squalene-based oil-in-water adjuvant” and “VLP”) throughout the experiment (p>0.05). The results are presented in Figure а and b.
Figure. Average weights of male (a) and female (b) mice in the study groups before the study – day 0, on days 1, 2, 3, 4, 7, 8 and 15 after vaccine administration, g (m ± SEM). Average weights of male (c) and female (d) rats in the study groups before the study – day 0, on days 8, 15, 22, 29, 36, 43, 50 and 58 after vaccine administration, g (m ± SEM).
Vertical axis – animal weight, g; horizontal axis – experiment duration, days.
Рисунок. Средние значения массы самцов (а) и самок (б) мышей в исследуемых группах до исследования – 0-е сутки, на 1, 2, 3, 4, 7, 8 и 15-е сутки после введения вакцины, г (m ± SEM). Средние значения массы самцов (в) и самок (г) крыс в исследуемых группах до исследования – 0-е сутки, на 8, 15, 22, 29, 36, 43, 50 и 58-е сутки после введения вакцины, г (m ± SEM).
По вертикали – масса животных, г; по горизонтали – продолжительность эксперимента, сутки.
For the pathomorphologic evaluation of organs, necropsy with organ extraction was performed. There was no effect of the vaccine on the state of internal organs of mice at macroscopic examination. No significant both intragroup and intergroup differences were found. The location of internal organs was physiologically correct, natural orifices without peculiarities, and no free fluid was found. Also, no negative effect of the vaccine on the anatomy and weight of the organs was found. The obtained results of relative weight of internal organs are presented in Table 1.
Table 1. Relative mass of internal organs of mice, % (m ± SEM)
Таблица 1. Относительная масса внутренних органов самцов и самок мышей, % (m ± SEM)
Organ Орган | Group Группа | |||||
NaCl 0.9% Физ. р-р | Adjuvanted with an oil-in-water Адьювант на основе сквалена | VLP | ||||
♀, n = 10 | ♂, n = 10 | ♀, n = 10 | ♂, n = 10 | ♀, n = 10 | ♂, n = 10 | |
Brain Головной мозг | 1.2 ± 0.06 | 1.1 ± 0.06 | 1.3 ± 0.05 | 1.0 ± 0.04 | 1.3 ± 0.09 | 1.1 ± 0.08 |
Thymus Тимус | 0.2 ± 0.03 | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.01 |
Heart Сердце | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.02 |
Lungs Легкие | 0.8 ± 0.11 | 0.7 ± 0.07 | 0.7 ± 0.06 | 0.9 ± 0.11 | 0.9 ± 0.11 | 0.7 ± 0.12 |
Kidneys Почки | 1.1 ± 0.04 | 1.2 ± 0.04 | 1.1 ± 0.04 | 1.3 ± 0.03 | 1.1 ± 0.04 | 1.3 ± 0.06 |
Adrenal glands Надпочечники | 0.04 ± 0.002 | 0.02 ± 0.001 | 0.03 ± 0.002 | 0.02 ± 0.002 | 0.04 ± 0.002 | 0.02 ± 0.001 |
Liver Печень | 3.8 ± 0.13 | 3.6 ± 0.06 | 3.8 ± 0.14 | 3.6 ± 0.07 | 3.6 ± 0.10 | 3.8 ± 0.09 |
Spleen Селезенка | 0.4 ± 0.07 | 0.3 ± 0.02 | 0.4 ± 0.08 | 0.3 ± 0.02 | 0.4 ± 0.07 | 0.4 ± 0.03 |
Thymus/spleen Тимус/селезенка | 0.5 ± 0.12 | 0.5 ± 0.05 | 0.5 ± 0.06 | 0.5 ± 0.05 | 0.5 ± 0.10 | 0.5 ± 0.05 |
Testes/Ovaries Семенники/яичники | 0.1 ± 0.01 | 0.6 ± 0.04 | 0.1 ± 0.01 | 0.7 ± 0.03 | 0.2 ± 0.01 | 0.7 ± 0.05 |
Note. Here and in the table. 2, 3 data are presented as group means with standard errors of the mean (m ± SEM).
Примечание. Здесь и в табл. 2, 3 данные представлены в виде средних значений по группе со стандартными ошибками среднего (m ± SEM).
In the study of subchronic toxicity on rats, no death of animals was registered. Monitoring of general condition and behavior throughout the experiment was carried out daily. No deviations were revealed; the Open Field test was without peculiarities. Reaction to external stimuli, orientation in space, muscle tone and coordination of movements were within physiological norms. There were no signs of aggression. The rats looked healthy, pelt was without peculiarities, skin – without pathological signs. The abdomen showed no signs of enlargement; visible mucous membranes – moderately moist, pink, clean; teeth – without changes; ears and eyes – without inflammatory signs; lacrimation and salivation – without disorders. Breathing is even, without peculiarities. The amounts of urination and defecation were in accordance with the physiological norm; there was no nasal discharge. In certain animals after three times daily instillation of both vaccine and carrier into the nasal cavity there appeared redness and swelling of the nasal speculum. The described phenomena disappeared on their own after 24 hours. Thermometry of animals did not reveal any deviations beyond the physiological norm [15, 16]. There was no significant effect of intranasal administration of VLP vaccine, both in vaccination and double vaccination doses, on the dynamics of body weight of rats, which shows that there is no negative effect of VLP vaccine on this index (Fig. c and d).
Group mean values of clinical blood analysis are presented in Table 2. One day after the last immunization (43rd day), a significant increase of less than 2-fold in the number of platelets was detected in male rats receiving 160 µg of antigen per dose (double inoculation dose) compared to the control group. The observed increase in platelet counts was reversible and was not observed in other experimental groups or at other times of clinical blood tests throughout the study. After 14 days there were no statistically significant differences of this index between the experimental and control groups. At the same time, there were no significant differences between the groups for the rest of the studied blood analysis parameters, which did not exceed the limits of physiological norms for rats [15, 16].
Table 2. Clinical blood test parameters of rats on the 1st and 14th days after the last immunization (43th and 58th days of the experiment, respectively), % (m ± SEM)
Таблица 2. Показатели клинического анализа крови самцов и самок крыс на 1-е и 14-е сутки после последней вакцинации (43 и 58-е сутки эксперимента соответственно), % (m ± SEM)
Blood count Показатель | Days Сутки | Group Группа | |||||
Adjuvanted with an oil-in-water Адьювант на основе сквалена | VLP 80 μg VLP 80 мкг | VLP 160 μg VLP 160 мкг | |||||
♀, n = 15 | ♂, n = 15 | ♀, n = 15 | ♂, n = 15 | ♀, n = 15 | ♂, n = 15 | ||
RBC, 1012/l RBC, 1012/л | 43 | 7.8 ± 0.11 | 7.9 ± 0.37 | 7.7 ± 0.33 | 8.3 ± 0.20 | 7.9 ± 0.30 | 8.3 ± 0.19 |
58 | 8.0 ± 0.26 | 8.7 ± 0.23 | 7.8 ± 0.14 | 8.7 ± 0.27 | 7.6 ± 0.12 | 8.2 ± 0.42 | |
PLT, 109/l PLT, 109/л | 43 | 374.6 ± 28.01 | 355.7 ± 49.87 | 417.2 ± 32.29 | 363.2 ± 28.48 | 517.6 ± 70.96 | 600.4 ± 67.74* |
58 | 347.2 ± 10.84 | 338.0 ± 16.10 | 365.2 ± 7.89 | 369.2 ± 5.15 | 330.0 ± 10.50 | 372.4 ± 7.55 | |
HCT, % | 43 | 43.0 ± 0.69 | 41.6 ± 1.75 | 41.5 ± 1.72 | 43.9 ± 1.00 | 42.9 ± 1.63 | 44.0 ± 1.08 |
58 | 43.3 ± 1.04 | 46.1 ± 1.18 | 42.2 ± 1.06 | 45.0 ± 1.29 | 41.7 ± 0.47 | 43.9 ± 2.02 | |
HGB, g/l HGB, г/л | 43 | 147.3 ± 2.58 | 139.4 ± 6.10 | 141.8 ± 6.04 | 147.1 ± 3.46 | 146.1 ± 5.87 | 144.0 ± 7.91 |
58 | 149.2 ± 3.83 | 160.0 ± 4.45 | 146.7 ± 4.14 | 154.3 ± 5.18 | 144.2 ± 2.06 | 144.7 ± 7.02 | |
WBC, 109/l WBC, 109/л | 43 | 10.9 ± 1.72 | 10.1 ± 0.99 | 9.9 ± 1.11 | 9.0 ± 1.20 | 9.8 ± 1.09 | 11.0 ± 1.54 |
58 | 11.0 ± 1.72 | 17.5 ± 1.60 | 11.0 ± 1.55 | 12.2 ± 2.40 | 15.0 ± 1.19 | 15.7 ± 0.72 | |
LYM, % | 43 | 63.1 ± 1.90 | 64.4 ± 0.95 | 67.7 ± 0.99 | 67.3 ± 1.73 | 65.8 ± 1.93 | 64.9 ± 1.78 |
58 | 64.5 ± 2.32 | 57.3 ± 2.20 | 62.0 ± 1.18 | 53.5 ± 3.24 | 63.8 ± 5.59 | 56.5 ± 5.37 | |
MON, % | 43 | 4.1 ± 0.27 | 3.8 ± 0.16 | 3.7 ± 0.18 | 3.3 ± 0.14 | 4.0 ± 0.35 | 3.7 ± 0.17 |
58 | 4.1 ± 0.86 | 4.0 ± 0.35 | 4.5 ± 0.34 | 3.9 ± 0.22 | 3.8 ± 0.27 | 4.0 ± 0.33 | |
GRAN, % | 43 | 32.8 ± 1.90 | 31.9 ± 0.87 | 28.6 ± 0.88 | 29.4 ± 1.66 | 30.2 ± 1.85 | 31.5 ± 1.67 |
58 | 31.4 ± 2.25 | 38.7 ± 2.34 | 31.7 ± 0.90 | 39.9 ± 3.42 | 28.4 ± 8.36 | 36.5 ± 4.42 | |
Note. RBC – erythrocytes; PLT – platelets; HCT – hematocrit; HGB – hemoglobin; WBC – leukocytes; LYM – lymphocytes; MON – monocytes; GRAN – granulocytes. * – statistically significant difference compared to the control according to Student’s t-test (p < 0.05).
Примечание. RBC – эритроциты; PLT – тромбоциты; HCT – гематокрит; HGB – гемоглобин; WBC – лейкоциты; LYM – лимфоциты; MON – моноциты; GRAN – гранулоциты. * – статистически значимое различие в сравнении с контролем по t-критерию Стьюдента (р < 0,05).
The study of the hemostasis system by such coagulogram indicators as activated partial thromboplastin time (APTT), recalcification time and fibrinogen did not reveal significant differences between the experimental and control groups. Blood biochemical parameters (total protein, cholesterol, triglycerides, total bilirubin, aspartate and alanine aminotransferase, alkaline phosphatase, urea) and urinalysis (protein, ketones, specific gravity, pH) in rats receiving intranasal vaccine based on VLP inoculated and double inoculated dose were not statistically significantly different from similar parameters in rats of control groups. No signs of intoxication and pathologic abnormalities in the structure of internal organs were detected in male and female rats. The average values of relative weight of internal organs of rats are presented in Table 3.
Table 3. Relative mass of internal organs of rats on the 1st and 14th days after the last immunization (43th and 58th days of the experiment, respectively), % (m ± SEM)
Таблица 3. Морфометрический анализ внутренних органов самцов и самок крыс на 1-е и 14-е сутки после последней вакцинации (43 и 58-е сутки эксперимента соответственно), % (m ± SEM)
Organ Орган | Days Сутки | Group Группа | |||||
Adjuvanted with an oil-in-water Адьювант на основе сквалена | VLP 80 μg VLP 80 мкг | VLP 160 μg VLP 160 мкг | |||||
♀, n = 15 | ♂, n = 15 | ♀, n = 15 | ♂, n = 15 | ♀, n = 15 | ♂, n = 15 | ||
Brain Головной мозг | 43 | 0.6 ± 0.02 | 0.4 ± 0.01 | 0.6 ± 0.01 | 0.4 ± 0.02 | 0.6 ± 0.02 | 0.4 ± 0.01 |
58 | 0.6 ± 0.03 | 0.4 ± 0.02 | 0.6 ± 0.03 | 0.4 ± 0.01 | 0.6 ± 0.02 | 0.5 ± 0.02 | |
Thymus Тимус | 43 | 0.13 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
58 | 0.10 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.003 | 0.1 ± 0.01 | 0.1 ± 0.01 | |
Heart Сердце | 43 | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.004 |
58 | 0.3 ± 0.01 | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.0 ± 0.01 | 0.3 ± 0.02 | |
Lungs Легкие | 43 | 0.6 ± 0.05 | 0.4 ± 0.03 | 0.6 ± 0.05 | 0.5 ± 0.03 | 0.5 ± 0.05 | 0.4 ± 0.03 |
58 | 0.5 ± 0.03 | 0.5 ± 0.04 | 0.5 ± 0.05 | 0.4 ± 0.04 | 0.5 ± 0.03 | 0.6 ± 0.02* | |
Kidneys Почки | 43 | 0.6 ± 0.01 | 0.6 ± 0.02 | 0.6 ± 0.02 | 0.6 ± 0.02 | 0.6 ± 0.01 | 0.5 ± 0.01 |
58 | 0.6 ± 0.02 | 0.6 ± 0.03 | 0.6 ± 0.01 | 0.6 ± 0.01 | 0.6 ± 0.03 | 0.6 ± 0.02 | |
Adrenal glands Надпочечники | 43 | 0.02 ± 0.002 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 |
58 | 0.02 ± 0.003 | 0.01 ± 0.001 | 0.02 ± 0.002 | 0.01 ± 0.001 | 0.02 ± 0.002 | 0.01 ± 0.001 | |
Liver Печень | 43 | 2.4 ± 0.05 | 2.5 ± 0.08 | 2.4 ± 0.06 | 2.4 ± 0.06 | 2.4 ± 0.06 | 2.3 ± 0.04 |
58 | 2.5 ± 0.05 | 2.4 ± 0.10 | 2.5 ± 0.13 | 2.5 ± 0.13 | 2.8 ± 0.13 | 2.3 ± 0.05 | |
Spleen Селезенка | 43 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 |
58 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0.004 | 0.3 ± 0.03* | 0.2 ± 0.02 | |
Testes/Ovaries Семенники/яичники | 43 | 0.04 ± 0.004 | 0.7 ± 0.02 | 0.04 ± 0.002 | 0.7 ± 0.03 | 0.04 ± 0.002 | 0.7 ± 0.02 |
58 | 0.04 ± 0.002 | 0.7 ± 0.03 | 0.04 ± 0.005 | 0.7 ± 0.01 | 0.04 ± 0.001 | 0.8 ± 0.03 | |
Note. * ‒ statistically significant difference compared to the control according to Student’s t-test (p < 0.05).
Примечание. * ‒ статистически значимое различие в сравнении с контролем по t-критерию Стьюдента (р < 0,05).
In the experimental group of rats injected intranasally with VLP vaccine in a double vaccination dose (160 µg of antigen per dose), a one and a half times increase in the relative weight of spleens in females and lungs in males was detected compared to the control group. Although the increase was statistically significant, the values did not exceed the limits of physiologic norms for laboratory rats [15, 16].
Visual assessment, cytologic and histologic analysis of the vaccine injection site showed no local irritant effect.
Discussion
Despite limited data on the safety of SARS-CoV-2 vaccines, their application in pandemic settings was necessary. However, the subsequent development of adverse events and severe complications in volunteers adversely affects public confidence in vaccination. Bibliometric analysis of 1312 COVID-19 vaccine studies by Y. Chen et al. showed that they primarily focused on clinical trials [17], while preclinical safety studies were hardly covered at all.
Previously, approved VLP-based vaccines against human papillomavirus (Gardasil, Gardasil9, Cervarix), hepatitis B (Sci-B-Vac), hepatitis E (Hecolin) and malaria (Mosquirix) have been shown to be safe, immunogenic and effective [18]. All the mentioned vaccines are administered intramuscularly, as are seven VLP vaccines against COVID-19, which are in various stages of clinical trials1. The development of vaccines against SARS-CoV-2 with an intranasal route of administration is increasingly attracting the interest of researchers from different countries and, despite the variety of possible platforms, they are predominantly vector vaccines [19]. The development of polyvalent vaccines for COVID-19 prophylaxis effective against new variants of SARS-CoV-2 is still an urgent task [20]. One of the important stages of vaccine development is safety studies, including the determination of acute and chronic toxicity; therefore, preclinical safety studies of previously developed, obtained and characterized VLPs were conducted [21] for intranasal route of administration.
In accordance with the Guidelines for conducting preclinical studies of drugs edited by A.N. Mironov [10], animal studies provide the most complete information on the toxic properties of the vaccine under test, which is supposed to be used in humans. Mice and rats are standard objects of toxicological studies in the number sufficient for complete registration of the studied effects and statistical processing of the obtained data.
This study presents the results of acute (mice) and subchronic (rats) toxicity studies with evaluation of local tolerance, conducted on outbred animals (to exclude genotypic dependence), of both sexes, with a weight variation of less than 10% and a number sufficient for statistical processing of the results, but not exceeding ethically permissible numbers for the formation of experimental and control groups.
In the acute toxicity study, mice were injected once intranasally with VLP vaccine with a concentration of 200 mg of antigen per dose, which is more than 2000 times higher than the vaccination dose for humans. The investigated vaccine was non-toxic, there were no signs of intoxication and death of animals, and therefore the calculation of lethal doses was impossible. The results obtained coincide with the data obtained by other researchers [22, 23], they were also unable to calculate lethal doses due to the absence of mortality of the animals studied. This confirms the low toxicity of VLP-based vaccines.
In preclinical subchronic toxicity studies, rats were administered a single vaccine dose calculated for humans (80 µg) and a doubled dose (160 µg). It is noteworthy that, as in the case of the study on mice, there were no differences in the studied parameters between the groups. At the same time, in case of administration of a double inoculation dose of the drug to rats a day after the last immunization (43rd day), a passing statistically significant increase in the number of platelets less than 2-fold was observed in males, and on the 58th day an almost 1.5-fold increase in spleens in females and lungs in males was detected, but since these values did not exceed the physiological norms for laboratory rats [15, 16], the obtained deviations can be attributed to fluctuation changes not related to vaccination. Visual assessment, cytologic and histologic analysis of the vaccine injection site showed no local irritant effect. Preclinical studies presented by Iranian researchers showed that subunit vaccine with heterologous prime-boost vaccination method, when animals were administered two doses of vaccine intramuscularly and one dose intranasally, showed no changes in general clinical observations; body weight and food consumption, clinical parameters, hematologic examination, blood chemistry analysis and pathologic examination of vital organs. Vaccine safety has been established after administration of single and repeated dose of the drug [23].
Continuous evaluation of vaccine safety is essential and the results should be disseminated to build confidence in immunization programs to increase public commitment to vaccine prophylaxis [24].
Intranasal immunization has demonstrated a promising ability to stimulate mucosal secretory immunity along with the induction of humoral and cellular immunity, and has the added advantage of ease of administration and dosing compared with parenteral forms of administration.
The safety demonstrated in the present study in acute and subchronic toxicity studies of a quadrivalent VLP-based vaccine for the prevention of COVID-19 intranasal administration allows for further development of this drug product in extensive preclinical and follow-up clinical studies.
Conclusion
The presented study of acute and subchronic toxicity with evaluation of local tolerability of quadrivalent VLP vaccine against COVID-19 is a part of preclinical studies of safety, immunogenicity and efficacy of intranasal VLP-based vaccine against COVID-19. The concept and design of the study were developed taking into account the requirements of the Ministry of Health of the Russian Federation, standards in the field of preclinical safety studies of new pharmacological agents – GLP (Good Laboratory Practice) system and guidelines for conducting preclinical studies of medicines edited by A.N. Mironov.
Both an acute toxicity study in outbred mice and a subchronic toxicity study in outbred rats demonstrated the safety of a quadrivalent VLP-based vaccine for COVID-19 prophylaxis. It was shown that nasal instillations (vaccination dose, double vaccination dose and antigen dosage more than 2000 times higher than the human vaccination dose) had no toxic effect on the organism of laboratory animals – rats and mice. The observed insignificant changes were transient and did not exceed the limits of physiological norms.
1 https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines, дата обращения 05.11.2024
2 National standard of the Russian Federation (GOST 33044-2014) «Principles of Good Laboratory Practice».
3 National standard of the Russian Federation GOST R 56701-2015 dated 01.07.2016 «Medicines for medical use. Guidelines for planning preclinical safety studies for the purpose of subsequent clinical trials and registration of medicinal products».
About the authors
Yana Y. Chernoryzh
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Author for correspondence.
Email: revengeful_w@mail.ru
ORCID iD: 0000-0001-9848-8515
SPIN-code: 3576-8760
Scopus Author ID: 57203299151
ResearcherId: AAI-7206-2020
PhD, Candidate of Medical Sciences, Researcher, laboratory of molecular diagnostics
Russian Federation, 123098, MoscowValeria M. Kondratieva
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: 1999valeriak@mail.ru
ORCID iD: 0000-0001-9163-4516
graduate student, laboratory of molecular diagnostics
Russian Federation, 123098, MoscowАnastasia P. Malkova
Institute for Biomedical Research and Technology (IMBIIT)
Email: nastena0302@yandex.ru
ORCID iD: 0000-0002-2817-4817
head of the Laboratory of Biological Research
Russian Federation, 143090, Krasnoznamensk, Moscow regionTatyana E. Savochkina
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: tasavochkina@yandex.ru
ORCID iD: 0000-0003-4366-8476
Researcher, Laboratory of Molecular Diagnostics
Russian Federation, 123098, MoscowOlesya V. Eliseeva
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: olesenka80@mail.ru
ORCID iD: 0000-0002-0723-9749
PhD, Senior Scientist, Laboratory of molecular diagnostics
Russian Federation, 123098, MoscowOleg E. Latyshev
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: oleglat80@mail.ru
ORCID iD: 0000-0002-5757-3809
PhD, Senior Scientist, Laboratory of molecular diagnostics
Russian Federation, 123098, MoscowDmitriy Y. Yakunin
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: yd364@mail.ru
ORCID iD: 0009-0009-4531-5739
graduate student of laboratory of molecular diagnostics
Russian Federation, 123098, MoscowOlga N. Zaykova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: zaykova_o_n@mail.ru
ORCID iD: 0000-0003-4708-2069
PhD, Senior Scientist at the Laboratory of Molecular Diagnostics
Russian Federation, 123098, MoscowEkaterina S. Sludnyakova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: ekaterina.ses@mail.ru
ORCID iD: 0009-0000-4925-5205
Leading engineer for the implementation of scientific developments
Russian Federation, 123098, MoscowTatyana V. Grebennikova
N.F. Gamaleya National Research Center of Epidemiology and Microbiology of the Ministry of Health of Russia
Email: t_grebennikova@mail.ru
ORCID iD: 0000-0002-6141-9361
Doctor of Biological Sciences, Professor, Corresponding Member RAS, deputy Director for Science of the Division of the Ivanovsky Virology Institute Head of the Control Center
Russian Federation, 123098, MoscowReferences
- Fernandes Q., Inchakalody V.P., Merhi M., Mestiri S., Taib N., Moustafa Abo El-Ella D., et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022; 54(1): 524–40. https://doi.org/10.1080/07853890.2022.2031274
- Grebennikova T.V., Eliseeva O.V., Latyshev O.E., Savochkina T.E., Tsibezov V.V., Cherepushkin S.A., et al. Recombinant virus-like particles for induction of specific immunity against severe acute respiratory syndrome virus SARS-CoV-2. Patent RU 2769224 C1; 2022. https://elibrary.ru/prmwjl (in Russian)
- Grebennikova T.V., Eliseeva O.V., Latyshev O.E., Cherepushkin S.A., Tsibizov V.V., Lebedeva V.V., et al. Virus-like chimeric particles for the induction of specific immunity against the severe acute respiratory syndrome virus SARS-CoV-2, containing proteins of coronavirus and rotavirus. Patent RU 2779810 C1; 2022. (in Russian)
- Gus’kova T.A., Syubaev R.D. Toxicological Aspects of Simultaneous Use of Various Medicines. Toxicology of Medicines [Toksikologicheskie aspekty odnovremennogo ispol’zovaniya razlichnykh lekarstvennykh sredstv. Toksikologiya lekarstvennykh sredstv]. Moscow: Russkii vrach; 2003; 116–40. (in Russian)
- Zapadnyuk I.P., Zapadnyuk V.I., Zakhariya E.A. Laboratory Animals, their Breeding and Use in Experiment [Laboratornye zhivotnye, ikh razvedenie i ispol’zovanie v eksperimente]. Kiev; 1982. (in Russian)
- International recommendations for conducting biomedical research using animals. Lanimagologiya. 1993; (1): 29. (in Russian)
- Menshikov V.V. Laboratory Research Methods in the Clinic [Laboratornye metody issledovaniya v klinike]. Moscow; 1987. (in Russian)
- Nazarenko G.I., Kishkun A.A. Clinical Evaluation of Laboratory Research Results: A Practical Guide [Klinicheskaya otsenka rezul’tatov laboratornykh issledovanii: Prakticheskoe rukovodstvo]. Moscow: Meditsina; 2007. (in Russian)
- Trakhtenberg I.M. Problems of Norm in Toxicology [Problemy normy v toksikologii]. Moscow; 1991. (in Russian)
- Mironov A.N. ed. Guidelines for Conducting Preclinical Studies of Medicines [Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekarstvennykh sredstv]. Moscow: Grif and K; 2012. (in Russian)
- Mironov A.N., Merkulov V.A. Guidelines for the Examination of Medicines. Volume 3 [Rukovodstvo po ekspertize lekarstvennykh sredstv. Tom 3]. Moscow: Poligraf-Plyus; 2014. (in Russian)
- Garber D.S., Barbi R.V., Bilitski D.T., Kleiton Li.E., Donovan D.K., Kon D.F., et al. Guidelines for the Maintenance and Use of Laboratory Animals [Rukovodstvo po soderzhaniyu i ispol’zovaniyu laboratornykh zhivotnykh]. Moscow: IRBIS; 2017. https://elibrary.ru/zrjvdj (in Russian)
- WHO. Guidelines on the non-clinical evaluation of vaccine adjuvants and adjuvanted vaccines; 2014. Available at: https://who.int/publications/m/item/nonclinical-evaluation-of-vaccine-adjuvants-and-adjuvanted-vaccines-annex-2-trs-no-987
- Requirements of the International Committee for Science on the use of laboratory animals in experimental research. Byulleten’ IKLAS. 1978; (24): 4–5. (in Russian)
- Lineva A. Physiological Indicators of the Norm of Animals. Reference Book [Fiziologicheskie pokazateli normy zhivotnykh. Spravochnik]. Moscow: Aquarium LTD; 2003. (in Russian)
- Abrashova T.V., Gushchin Ya.A., Kovaleva M.A., Rybakova A.V., Selezneva A.I., Sokolova A.P., et al. Guide. Physiological, Biochemical and Biometric Indicators of the Norm of Experimental Animals [Spravochnik. Fiziologicheskie, biokhimicheskie i biometricheskie pokazateli normy eksperimental’nykh zhivotnykh]. St. Petersburg: LEMA; 2013. https://elibrary.ru/ptsruo (in Russian)
- Chen Y., Cheng L., Lian R., Song Z., Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci. Trends. 2021; 15(2): 64–73. https://doi.org/10.5582/bst.2021.01061
- Nooraei S., Bahrulolum H., Hoseini Z.S., Katalani C., Hajizade A., Easton A.J., et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology. 2021; 19(1): 59. https://doi.org/10.1186/s12951-021-00806-7
- Bai Z., Wan D., Lan T., Hong W., Dong H., Wei Y., et al. Nanoplatform based intranasal vaccines: current progress and clinical challenges. ACS Nano. 2024; 18(36): 24650–81. https://doi.org/10.1021/acsnano.3c10797
- Marks P.W., Gruppuso P.A., Adashi E.Y. Urgent need for next-generation COVID-19 vaccines. JAMA. 2023; 329(1): 19–20. https://doi.org/10.1001/jama.2022.22759
- Latyshev O.E., Zaykova O.N., Eliseeva O.V., Savochkina T.E., Chernoryzh Ya.Yu., Syroeshkin A.V., et al. Development, production and characterization of SARS-CoV-2 virus-like particles (Coronaviridae: orthocoronavirinae: betacoronavirus: sarbecovirus). Voprosy virusologii. 2024; 69(2): 175–86. https://doi.org/10.36233/0507-4088-226 https://elibrary.ru/gkxfed (in Russian)
- Banihashemi S.R., Es-Haghi A., Fallah Mehrabadi M.H., Nofeli M., Mokarram A.R., Ranjbar A., et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: A preclinical study in several animal models. Front. Immunol. 2022; 13: 836745. https://doi.org/10.3389/fimmu.2022.836745
- Vakhrusheva A.V., Kudriavtsev A.V., Kryuchkov N.A., Deev R.V., Frolova M.E., Blagodatskikh K.A., et al. SARS-CoV-2 subunit virus-like vaccine demonstrates high safety profile and protective efficacy: preclinical study. Vaccines (Basel). 2022; 10(8): 1290. https://doi.org/10.3390/vaccines10081290
- Antonova N.A., Yeritsyan K.Yu. The systematic review of empirical research of factors of refusal from vaccination. Gigiena i Sanitaria (Hygiene and Sanitation, Russian journal). 2018; 97(7): 664–70. https://doi.org/10.18821/0016-9900-2018-97-7-664-670 https://elibrary.ru/uxaexo (in Russian)
Supplementary files