A simple, highly sensitive and specific serological test for the detection of antibodies to Varicella-zoster virus (Varicellovirus humanalpha3)
- Authors: Nagieva F.G.1, Barkova E.P.1, Kharchenko O.S.1, Sidorov A.V.1, Alatortseva G.I.1, Cherepovich B.S.1, Tarakanova Y.N.1, Trubacheva O.A.1, Pashkov E.A.1,2, Rtishchev A.A.1, Svitich O.A.1,2, Zverev V.V.1,2
-
Affiliations:
- I. Mechnikov Research Institute of Vaccines and Sera
- Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University)
- Issue: Vol 69, No 6 (2024)
- Pages: 489-499
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16675
- DOI: https://doi.org/10.36233/0507-4088-259
- EDN: https://elibrary.ru/ykzhop
- ID: 16675
Cite item
Abstract
Introduction. Varicella-Zoster virus (VZV) is a highly contagious alpha-herpes virus. The diagnosis of chickenpox remains a difficult task especially in cases of breakthrough chickenpox, so the development of reliable laboratory tests is necessary. The simplest and most sensitive serological test for detecting antibodies in human and animal sera is the passive hemagglutination reaction (PHAR).
The aim. To develop of a simple, highly sensitive and specific serological tests for the detection of antibodies to VZV in human and animal blood sera.
Materials and methods. Human and animal cell cultures; various strains of VZV; human and animal immune sera; monoclonal antibody to VZV glycoprotein (GP) E. Formalin-treated erythrocytes of sheep, chickens and goats, sensibilised with GP of VZV from a virus-containing supernatant were used for PHAR.
Results. Cell cultures with the maximum cytopathic effect at VZV infection were selected. A simple original method for obtaining virus-specific VZV GPs using lectins has been developed. Purified GPs were obtained by their elution from sheep erythrocytes after adsorption. The activity of VZV GP was confirmed in PHAR by an antibody diagnostic assay using formalin-treated sheep erythrocytes sensibilised using monoclonal antibodies to GP E of the “vOka” VZV strain (USA). Using GPs from different VZV strains, PHAR test and GP-based enzyme-linked immunosorbent assay (gpELISA) have been developed to detect antibodies in human and animal immune sera. These tests have high sensitivity, specificity and lack of cross-reactivity.
Conclusion. A highly specific, sensitive and reproducible tests for the detection of antibodies to VZV have been developed.
Full Text
Introduction
Varicella-Zoster virus (VZV) is a highly contagious alpha-herpesvirus that infects more than 90% of people worldwide [1, 2].
According to a report published by the World Health Organization in 2014, at least 140 million people fall ill with VZV each year, of which 4.2 million have severe complications leading to hospitalization and death [3]. VZV is mainly characterized by mild to moderate disease severity, but there is a high risk of severe VZV in pregnant women, newborns, VZV-seronegative adults, as well as immunocompromised individuals [4]. Approximately 1/3 of VZV survivors develop herpes zoster at the age of mostly over 50 years, usually accompanied by postherpetic neuralgia [5, 6].
In the era of universal vaccination against VZV, diagnosis of the disease on the basis of clinical symptoms is challenging, especially for the recent increase in cases of VZV outbreaks. The problem of accurate diagnosis of VZV infection in such cases can be solved by reliable laboratory tests [7]. In recent decades, a variety of serologic methods for detection of specific antibodies have been developed to aid diagnosis of VZV infection and have found application in epidemiologic studies, vaccine efficacy studies and disease risk assessment in health care workers [8]. The fluorescent antibody to membrane antigen assay (FAMA) and enzyme-linked immunosorbent assay (ELISA) based on VZV glycoproteins (GPs) (gpELISA), which are commercially unavailable in many countries, have the highest sensitivity [9]. A major and immunodominant viral protein in VZV virions and in infected cells is glycoprotein E (gpE), which is essential for virus replication and cell-to-cell transmission [10‒12]. Given the cell-associated nature of VZV, the FAMA test is considered a more reliable means of assessing protective immunity than the neutralization reaction (NR) [13].
Evaluation of the antigenic response to the four-component vaccine, measles, mumps, rubella and VZV (MMRV), plays a vital role in patient management. Until recently, most clinical laboratories used ELISA tests to detect IgG antibodies to MMRV antigens. However, the Advisory Committee on Immunization Practices (ACIP) and the US Centers for Disease Control and Prevention (CDC) do not recommend their use to assess vaccine-induced immunity against VZV due to low sensitivity [14, 15]. For high-throughput clinical laboratories, a more reliable, faster, and more sensitive technology than ELISA, a high-throughput multiplex automation technology for the detection of antibodies against MMRV antigens (BioPlex 2200, Bio-Rad), has recently been introduced that allows simultaneous detection of serum IgG antibodies to all 4 viruses in a single reaction [16].
Another most simple and highly sensitive test for the detection of antibodies in human and animal sera is passive hemagglutination reaction (PHAR). This test is used to detect antibodies to measles, rubella, foot and mouth disease, adenovirus, cytomegalovirus, human immunodeficiency virus, etc. [17‒19].
The aim of this study is to develop PHAR assay based on formalin-treated erythrocytes sensitized with VZV GPs.
Materials and methods
Cell cultures. Cell cultures: MRC-5 – a strain of diploid human embryonic lung cells (American Type Culture Collection, ATCC); KM-27 – skin and muscle tissue of human embryo (collection of Chumakov FSC R&D IBP RAS, Moscow, Russia), Vero-CCL-81 – a line of transplantable green monkey kidney cells (ATCC); Vero-E6 – a clone of the Vero cell line (collection of Chumakov FSC R&D IBP RAS, Moscow, Russia); A549 – human carcinoma cell line (collection of I.I. Mechnikov Research Institute of Vaccines and Sera, Moscow, Russia); PTP – piglet testicles (collection of All-Union Scientific Research Institute of Veterinary Virology and Microbiology, Pokrov, Vladimir region, Russia); two types of mesenchymal stem cells from the collection of cell cultures of I.I. Mechnikov Research Institute of Vaccines and Sera. Cell cultures were grown in DMEM/F-12 nutrient medium with 10 mM HEPES, supplemented with 5 or 10% bovine serum (LLC SPE BIOHIMSERVICE, Vladimir, Russia).
Viruses. Vaccine strains of «vFiraVax» VZV virus, «vZelVax» herpes zoster virus (collection of I.I. Mechnikov Research Institute of Herpes Virus); Japanese strain of «vOka» VZV virus (CCL collection, USA); laboratory strain of «Ellen» VZV virus (CCL collection); wild variants of VZV virus isolated during an outbreak in a closed collective (collection of I.I. Mechnikov Research Institute of Vaccines and Sera). The viruses were grown in cell cultures in DMEM medium with increased glucose content, without serum.
Immune sera and monoclonal antibodies (MCAs). Human sera to herpes zoster virus taken during reactivation of postherpetic neuralgia: Zel.-1 and Nik.-2; blood sera from patients who had survived VZV (n = 25); guinea pig immune serum to antigens of cytomegalovirus (CMV) strain AD169. Thermolabile and thermostable inhibitors were removed from the sera by RDE (receptor dectroing enzyme, Denka Seiken, Japan) according to the manufacturer’s instructions. MCA to gpE of VZV virus – Varicella mab (Potency, ID, Lot: PR 101022c, USA). MCA to gpB and gpD of herpes simplex virus types 1 and 2, respectively.
Neutralization reactions. Neutralization was performed on Vero-CCL-81 cell culture grown on 24-well plates (Costar) in DMEM growth medium with 10% FBS. Twofold dilutions of immune virus-specific guinea pig sera to the «vFiraVax» VZV vaccine strains and «vZelVax» herpes zoster were prepared. An equal volume of 1000 doses of HADE50/0.1 ml virus was added to each 0.1 ml dilution of immune sera, the mixture was mixed vigorously and left to contact for 1.5 h at 36.5 °C in a CO2 incubator, stirring every 15 min. In 24-well plates with a monolayer of cells and with previously removed growth medium, 0.2 ml of the virus-serum mixture was added into each two wells and left to contact for 1.5 h in the incubator. Then, 0.8 ml of DMEM maintenance medium without serum was added to the wells. Two wells were left on the plate for virus dose control and cell control, culturing was continued for 7 days. The NR result was counted at 100% cell protection.
Obtaining formalin-treated red blood cells. To obtain formalin-treated lamb, chicken and goat red blood cells, blood was collected in Alsever’s solution. Erythrocytes were washed three times with cooled saline solution by centrifugation for 20 min at 1500 rpm. To the washed erythrocytes, a 2% formalin solution in 10-fold volume of saline solution containing 0.5% glucose was added, and the pH of the solution was adjusted with 1 M NaOH to 7.0‒7.2. Formalin-treated erythrocytes were kept at room temperature for 3‒4 days until complete sedimentation of erythrocytes, the supernatant was removed, an equal volume of saline solution was added to the sediment and stored at 2‒8 °C. Before sensitization, formalin-treated erythrocytes were tested for spontaneous agglutination.
Preparation of virus-specific GPs. VZV-sensitive cells were grown in 175 cm2 culture vials, infected with virus-containing fluid (VCF) and cultured for 7‒8 days until 70‒90% destruction of infected cells. The vials were incubated for a day or more at −70 °C, then their contents were thawed, cell debris was removed by centrifugation at 1500 rpm for 20 min, sucrose-gelatin stabilizer was added to the supernatant and left at −70 °C for storage until sensitization of erythrocytes. To extract viral GPs from VCF, phytohemagglutinin (PHA) at a concentration of 25 μg/ml and 50% each of formalin-treated lamb, chicken or goat erythrocyte suspensions were added at a ratio of 10 : 1 by volume and left at 4 °C for 20 h with periodic shaking to bind viral GPs to PHA on the erythrocyte surface. The suspensions were centrifuged at 1500 rpm for 20 min, and saline solution was added to the precipitates of viral GP-loaded formalin-treated erythrocytes. Elution of viral GPs from the surface of formalin-treated erythrocytes was carried out for 1 h at 37 °C. Erythrocytes were removed from the suspension by centrifugation at 1500 rpm for 20 min. Bovine serum albumin (BSA) was added to the obtained solution of viral GP as a stabilizer to a concentration of 1%. Protein concentration was determined on a NanoDrop 2000 instrument (Thermo Scientific, USA).
Production of antigenic diagnostic agent based on viral GPs. Formalin-treated erythrocytes were sensitized with virus-specific GPs obtained from different cell types infected with VZV strains. For this purpose, erythrocytes were resuspended in a 10-fold volume of chilled saline solution, precipitated by centrifugation at 1500 rpm for 20 min and a 50% suspension of erythrocytes in saline solution was prepared. Next, 1 volume of bidistilled water was combined in centrifuge tubes with 0.1 volume of 50% red blood cell suspension, 0.1 volume of viral GP solution, and 0.1 volume of 0.33% chromium chloride (CrCl3). The tubes were placed in a water bath for 1 h at 42 °C, then an equal volume of saline solution was added, mixed, and the sensitized erythrocytes were precipitated by centrifugation at 1500 rpm for 5 min. The sediment of sensitized erythrocytes was resuspended in saline solution containing 1% BSA, washed twice with saline solution containing 1% BSA, and a 2.5% suspension of sensitized formalin-fixed erythrocytes was prepared in the same solution for use in PHAR.
Preparation of anti-VZV MCA-based antibody diagnostic agent. To 0.1 ml of a suspension of 50% formalin-treated erythrocytes prewashed 3 times with saline solution, 0.1 ml of saline solution, 0.1 ml of MCA to gpE (USA) with a concentration of 318 µg/0.1 ml were added. The mixture was stirred and 0.3 mL of 0.33% chromium chloride solution and 10 μL of 0.05 M NaOH were added. The mixture was incubated in a water bath for 1 h at 42 °C, then washed three times with saline solution and prepared a 2% antibody diagnostic agent in saline solution.
PHAR for the detection of antibodies to VZV in animal and human sera. The reaction was carried out by micromethod in a volume of 150 μl on V-shaped plates. Each well of the plate was filled with 50 µl of saline solution containing 1% normal rabbit serum heated in a water bath at 65 °C for 30 min, 50 µl of test serum in dilutions from 1 : 100 to 1 : 12 400, and 50 µl of sensitized antigenic diagnostic agent. In 4 free wells separately added 50 µl of formalin-treated erythrocytes and 50 µl of antigenic diagnostic agent to control for the absence of spontaneous agglutination. The plate was left at 4 °C until complete sedimentation of control erythrocytes and antigenic diagnostic agent and then the reaction was counted. In the presence of antibodies specific to the viral antigen in the test material, erythrocytes were fixed at the bottom and walls of the well in the form of hemagglutination umbrella. In case of negative reaction erythrocytes settled to the bottom of the well in the form of a button.
PHAR for determination of VZV gpE activity. The wells of the V-plate were filled with 25 or 50 µl of 1% normal rabbit serum followed by 25 or 50 µl of 2% antibody diagnostic agent. The free wells were used separately for the spontaneous agglutination control of the diagnostic and the formalin-treated lamb erythrocyte control. The plate was incubated for 1.0‒1.5 h at 4 °C until the erythrocytes settled in the control wells and the reaction was taken into account.
Enzyme immunoassay. ELISA was carried out by the generally accepted method. At the first stage, the antigen solution was added 50 µl per well, incubated overnight at 4 °C, then the wells of the plate were blocked with casein-saccharose solution for 90 min and the plates were dried for 2 h in the thermostat at 37 °C with the door open. For ELISA, 50 µl of dilutions of human, mouse or guinea pig serum samples in phosphate buffered saline with tween (PBS-T) were added to the well in twofold increments, starting with a dilution of 1 : 100, incubated for 90 min at 37 °C; the plate was washed 3 times with PBS-T; 50 µl each of anti-human IgG conjugates were added at a working dilution of 1 : 5000, anti-mouse IgG Bio-Rad at a working dilution of 1 : 2000; anti-pig IgG in working dilution 1 : 5000 in PBS-T + 1% BSA, incubated the plates 60 min at 37 °C; the plates were washed 3 times with PBS-T; tetramethylbenzidine solution was added, incubated 15 min in a dark place; the reaction was stopped with sulfuric acid, the results were recorded on a spectrophotometer at a wavelength of 450 (comparison wavelength 630 nm).
Ethical approval. The authors confirm compliance with institutional and national standards for the use of laboratory animals in accordance with Consensus author guidelines for animal use (IAVES July 23, 2010). The study protocol was approved by the Ethical Committee of I.I. Mechnikov Research Institute of Vaccines and Sera (Protocol No. 8 of 13.08.2024).
Statistical methods. Excel 2013 (Microsoft, USA) was used for statistical processing of the results. Comparison of quantitative values of the obtained samples was performed using the nonparametric Mann‒Whitney U-test.
Results
At the first stage of development of the serologic test of PHAR, cell cultures were selected, during infection with VZV the maximum cytopathic effect (CPE) was determined, namely, KM-27, A549 and PTP cells, during infection with different strains of VZV 70‒90% cell death was observed on the 7‒8th day.
Next, the adsorption capacity of formalin-fixed animal erythrocytes, which are carries of the main components in PHAR, was determined. Three types of erythrocytes were selected: sheep, chicken and goat.
Lectins are known to specifically bind GPs of viruses [20, 21]. Two lectins, concavalin A (ConA) and PHA, were used to isolate virus-specific GPs from VCF. The blood serum of a guinea pig immunized with the «vZelVax» VZV vaccine strain was used in PHAR. The titer of guinea pig immune serum in NR on VZV-sensitive KM-27 cells was 1 : 6400 HADE50/0.2 ml.
Fig. 1 shows the results of determining the concentration of ConA and PHA lectins required for efficient binding of VZV GPs for subsequent sensitization of formalin-treated chicken erythrocytes. The optimal concentration of ConA and PHA was 25 μg/mL (p ≤ 0.05). At higher concentrations, serum titers in PHAR decreased 4-fold.
Fig. 1. Determination of the optimal concentration of ConA and PHA for binding virus-specific glycoproteins of VZV from virus-containing liquid.
Рис. 1. Определение оптимальной концентрации КонА и ФГА для связывания вирусоспецифических ГП VZV из вируссодержащей жидкости.
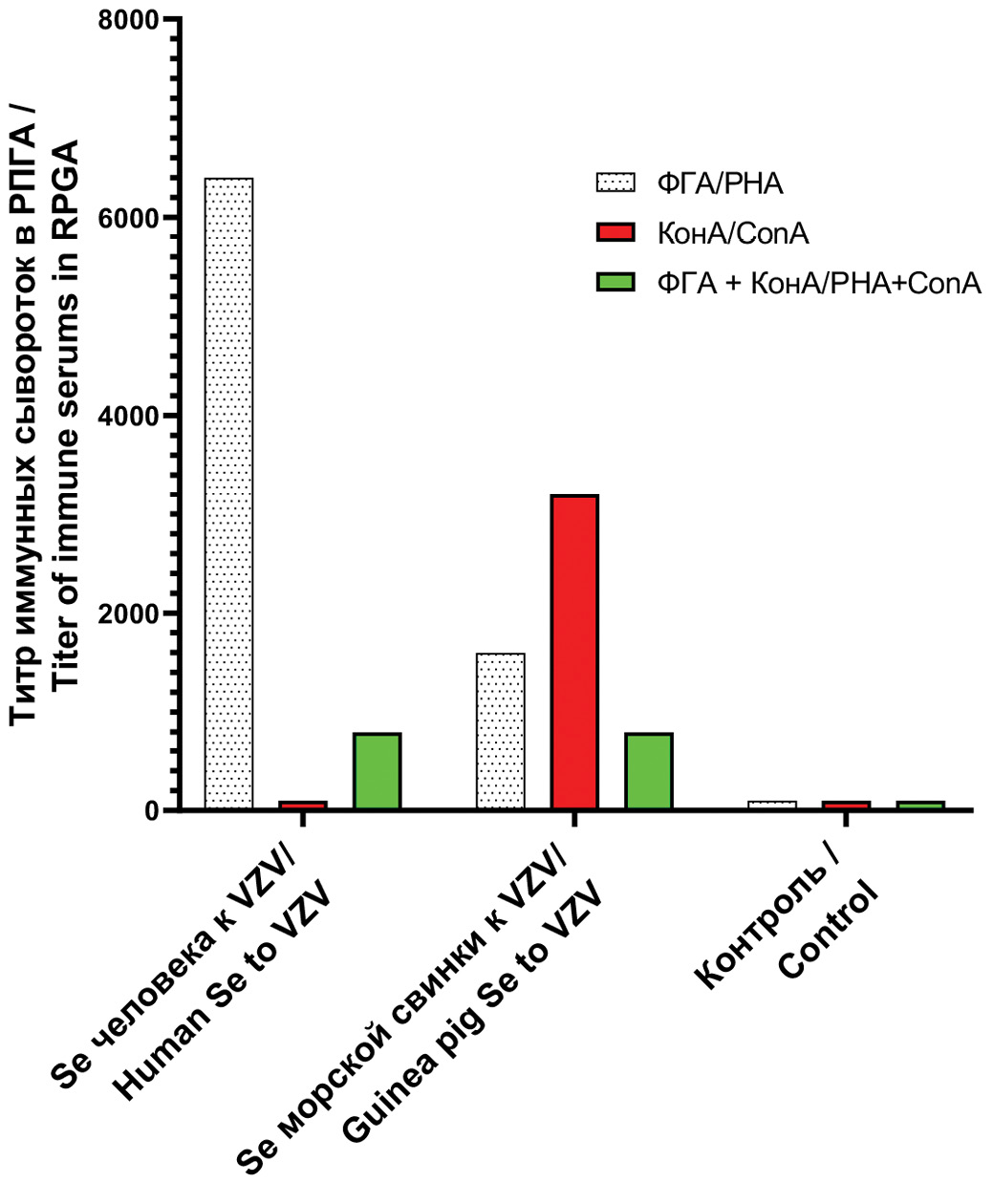
Fig. 2 shows the results of PHAR titration of human and guinea pig immune sera with sensitized viral GP antigenic diagnostics obtained with PHA, ConA and lectin mixture.
Fig. 2. Titer of immune sera in gpRPGA with antigenic diagnostics on formalized sheep erythrocytes, sensitized with viral glycoproteins obtained with various lectins.
Рис. 2. Титр иммунных сывороток в gpРПГА с антигенными диагностикумами на формализированных бараньих эритроцитах, сенсибилизированных вирусными ГП, полученными с различными лектинами.
The presented results clearly demonstrate that formalin-fixed sheep erythrocytes sensitized with PHA-derived viral GPs show virus-specific antibodies in human and animal sera in PHAR, with slightly reduced titers in animal sera.
PHAR using formalin-fixed sheep erythrocytes sensitized with ConA-derived viral GPs did not detect virus-specific antibodies in human sera. The use of a mixture of two lectins in equal concentrations to produce viral GPs from the same VCF reduces the level of antibody titers in PHAR.
The results of experiments with purified GPs eluted with formalin-fixed sheep erythrocytes are presented next. The titers of purified viral GPs were determined in gpPHAR using an antibody diagnostic agent prepared on lamb erythrocytes sensitized with MCA to gpE VZV (USA). The concentrations of viral GPs obtained by infection of cell cultures A549, KM-27, Vero-E6 and PTP with viral strains «vZelVax», «vFiraVax», «vOka», «Ellen», wild virus VZV (Moscow) were in the range from 0.206 to 0.381 mg/mL, the median titer for the obtained GPs in gpPHAR was 1 : 8.
The ability of formalin-treated sheep, chicken, and goat erythrocytes to adsorb viral GPs was determined to optimize gpPHAR conditions. GP sensitized VZV erythrocytes were used in PHAR to titrate serum from guinea pigs infected with the «vZelVax» VZV vaccine strain. The results showed that sheep erythrocytes had a higher sorption capacity (gpPHAR titer 1 : 3200, sedimentation rate 1.0‒1.5 h) in contrast to chicken (gpPHAR titer 1 : 800, sedimentation rate 20 min) and especially goat (sedimentation rate 1.0–1.5 h) erythrocytes. These data are in agreement with the results of other investigators obtained for other viral and bacterial agents [22, 23].
Fig. 3 shows the results of gpPHAR titrations of sera from individuals who had contracted herpes zoster and VZV, as well as sera from guinea pigs immunized with the «vZelVax» VZV vaccine strain, using formalin-treated chicken and sheep erythrocytes sensitized with herpes zoster virus GPs obtained using PHA lectin. The titers in gpPHAR were higher on the sheep erythrocytes in reactions with all serum samples used in the experiment. It can be concluded that the test is suitable for the detection of specific antibodies in both humans and animals.
Fig. 3. Comparative titration of human and guinea pig immune sera in gpRPGA using sensitized by GP VZV formalized chicken and lamb erythrocytes.
1 ‒ serum of patient with herpes zoster; 2 ‒ blood serum of children with chickenpox; 3 – blood serum of a guinea pig immunized with the «vZelVax» VZV vaccine strain.
Рис. 3. Сравнительное титрование иммунных сывороток человека и морской свинки в gpРПГА с применением сенсибилизированных ГП VZV формализированных куриных и бараньих эритроцитов,
1 ‒ сыворотка крови больного опоясывающим герпесом, полученная в период реактивации; 2 ‒ сыворотка крови детей, переболевших ветряной оспой; 3 ‒ сыворотка крови морской свинки, иммунизированной вакцинным штаммом «vZelVax» VZV.
The specificity of gpPHAR was established in reactions with human sera not containing antibodies to VZV. Given that 99.0% of the human population contains antibodies to VZV, an equal volume of viral GPs was added to the tested sera, and the mixture was incubated for 30 min at 37 °C to bind neutralizing antibodies. Fig. 4 shows the results of gpPHAR (Fig. 4 a) and gpELISA (Fig. 4 b) testing of 5 human sera treated and untreated with VZV GPs.
Fig. 4. Study on the specificity of immune sera in serological tests gpRPGA (а) and gpELISA (b).
* – termolabile and thermostable ingibitors of serological reactions have been preliminary removed from serums; ** – serological reactions have not been preliminary removed from serums.
Рис. 4. Исследование специфичности серологических тестов gpРПГА (а) и gpИФА (б).
* ‒ из сывороток предварительно удалены термолабильные и термостабильные ингибиторы серологических реакций; ** ‒ из сывороток предварительно не удалены ингибиторы серологических реакций.
The presented results clearly demonstrate the high specificity of gpPHAR compared to gpELISA: PHAR did not detect antibodies in any of the processed samples, whereas gpELISA detected antibodies in two immune sera, with antibody titers that were reduced by a factor of 4 and 8. Thus, partial cross-reactivity in gpELISA is noted. In general, there is a problem of cross-reactivity of serologic tests, especially ELISA, which can be partially or completely overcome by removing thermolabile and thermostable inhibitors of serologic reactions from sera. Fig. 5 shows the results of cross-reactivity testing of immune sera.
Fig. 5. Comparative titration of immune sera in RN (titer in GADE 50/0.5 ml), gpRPGA (titer in GAE 50/0.5 ml) and gpELISA (titer in conventional units).
Antibodies in serum: 1 ‒ «FiraVax», guinea pig (to alpha herpes virus type 3); 2 ‒ MKA-1H-110, mouse (to alpha herpes virus type 1); 3 ‒ MKA-2H-208, mouse (to alpha herpes virus type 2); 4 ‒ CMV-169, guinea pig (to beta herpes virus type 5).
Рис. 5. Сравнительное титрование иммунных сывороток в РН (титр в ГАДЕ 50/0,5 мл), gpРПГА (титр в ГАE 50/0,5 мл) и gpИФА (титр в условных единицах).
Антитела в сыворотке: 1 ‒ «FiraVax», морской свинки pig (к альфа-герпесвирусу типа 3); 2 ‒ МКА-1Н-110, мышиные (к альфа-герпесвирусу типа 1); 3 ‒ МКА-2Н-208, мышиные (к альфа-герпесвирусу типа 2); 4 ‒ ЦМВ-159, морской свинки (к бета-герпесвирусу типа 5).
The presented results show that in NR with 1000 HADE50/0.1 ml dose of VZV and in gpPHAR with antigenic diagnostics on GP sensitized VZV formalin-treated sheep erythrocytes only antibodies to herpesvirus type 3 are detected, namely to the «vFiraVax» VZV vaccine strain, and are undetectable to herpesviruses types 1, 2 and 5, in contrast to the gpIFA test, for which cross-immunoreactivity has been demonstrated.
Next, a comparative titration of 27 immune sera in gpPHAR, gpELISA and ELISA was performed. The results are summarized in Fig. 6.
Fig. 6. Results of comparative titration of immune sera to VZV in gpRPGA and gpELISA and ELISA.
Рис. 6. Результаты сравнительного титрования иммунных сывороток к VZV в gpРПГА, gpИФА и ИФА.
Analysis of the results of titration of immune sera in gpPHAR and gpELISA (Fig. 6) showed that 44.4% of sera had identical titers, sometimes differing by one dilution step. However, for 55.6% of the samples, the titers in gpPHAR were greater than those in gpELISA, and in no case did the titers in gpIFA exceed those in gpPHAR. Comparing the titers in gpELISA and conventional ELISA, 59.3% of cases had lower titers in gpPHAR than in ELISA, for 29.6% of samples the titers in gpELISA were equal to those in ELISA, and in 11.1% of cases the titers in gpELISA were greater than those in ELISA by one dilution step.
Discussion
The aim of the current study was to create a highly sensitive and specific simple serologic test with proven absence of cross-reactivity. The gpPHAR serologic test format meets these requirements. gpPHAR can only detect antibodies to VZV GPs, i.e. to neutralizing epitopes of viral antigens, which provide the main protective effect against VZV infections. Until now, it is believed that the infectivity of VZV remains closely associated with the cell and the newly formed virus is not released into the culture medium [24]; therefore, the gpPHAR test for detection of antibodies against VZV GP is more reliable than the NR. Since this test determines the level of antibodies directed exclusively to viral GPs, it is a reliable and sensitive indicator of immunity.
In foreign scientific literature there is a publication on the development of gpPHAR for the detection of antibodies to herpesviruses on the basis of viral GPs [20]. The authors obtained purified GPs of herpesviruses from VCF using lentil lectins sensitized on Sepharose 4 B (Pharmacia) and eluted with 0.2 M a-methyl mannoside. It should be noted that most commercial immunoassay test systems are not sensitive enough to detect post-vaccination antibodies [25].
We developed a simple and original method for the production of VZV GPs. VZV GPs were isolated from VCF of cell cultures infected with different strains of VZV by selective binding to the legume lectins ConA and PHA and sorption on formalin-treated sheep erythrocytes. The most effective lectin was PHA, which allowed the preparation of GPs for detection of virus-specific neutralizing antibodies in human and animal sera by gpPHAR. To obtain purified GPs, we used a well-known method of purification and concentration of viruses, in particular the influenza virus, which involved the adsorption of viruses on erythrocytes of chickens or rams at 4 °C and then elution from these erythrocytes at 37 °C [26]. The concentrations of viral GPs purified by the above method ranged from 0.206 to 0.381 mg/mL and their titers ranged from 1 : 8 using an erythrocyte antibody diagnostic agent. It was confirmed that of the three types of formalin-treated animal erythrocytes studied: sheep, chicken and goat erythrocytes, sheep erythrocytes have the highest adsorption capacity.
Comparative analysis showed that the developed gpPHAR test has high specificity and reproducibility. An important advantage in comparison with ELISA is the absence of cross-reactivity. Another clear advantage of this serologic test is its ease of performance, which allows its application in any laboratory.
Conclusion
A highly sensitive and specific, easy-to-perform, non-cross-reactive serologic gpPHAR test for detection of post-vaccination and post-infection antibodies to VZV has been developed. Its development is a stage in the production of a multiplex diagnostic kit for the detection of antibodies in the blood sera of children immunized with a four-component vaccine against measles, mumps, rubella and varicella zoster.
About the authors
Firaya G. Nagieva
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-8204-4899
D. Sci. (Med.) Associate Professor, Head of Laboratory of hybrid cell cultures, Department of virology
Russian Federation, 105064, MoscowElena P. Barkova
I. Mechnikov Research Institute of Vaccines and Sera
Author for correspondence.
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-3369-8869
Cand.Sci. (Biol.), leading researcher, Laboratory of hybrid cell cultures, Department of virology
Russian Federation, 105064, MoscowOlga S. Kharchenko
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-2169-9610
researcher, Laboratory of genetics of DNA-containing viruses, Department of virology
Russian Federation, 105064, MoscowAlexander V. Sidorov
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-3561-8295
Cand. Sci. (Biol.), Head of Laboratory of genetics of DNA-containing viruses, Department of virology
Russian Federation, 105064, MoscowGalina I. Alatortseva
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-9887-4061
Cand. Sci. (Biol.), Head of Laboratory of cloning of viruses genomes, Department of virology
Russian Federation, 105064, MoscowBogdan S. Cherepovich
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-5803-6263
Junior Researcher, Laboratory of genetics of RNA viruses
Russian Federation, 105064, MoscowYulia N. Tarakanova
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0003-3226-5989
Cand. Sci. (Biol.), Head of Laboratory of diagnostics of viral infections, Department of virology
Russian Federation, 105064, MoscowOlga A. Trubacheva
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0009-0005-0821-5553
leading specialist, Laboratory of hybrid cell cultures, Department of virology
Russian Federation, 105064, MoscowEvgeny A. Pashkov
I. Mechnikov Research Institute of Vaccines and Sera; Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University)
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-5682-4581
Junior Researcher, Laboratory of Virology Applied
Russian Federation, 105064, Moscow; 119048, MoscowArtem A. Rtishchev
I. Mechnikov Research Institute of Vaccines and Sera
Email: fgn42@yandex.ru
ORCID iD: 0000-0002-4212-5093
Researcher, Laboratory of genetics of RNA viruses
Russian Federation, 105064, MoscowOksana A. Svitich
I. Mechnikov Research Institute of Vaccines and Sera; Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University)
Email: fgn42@yandex.ru
ORCID iD: 0000-0003-1757-8389
D. Sci. (Med.), Corresponding Member of the Russian Acaqdemy of Sciences, Director
Russian Federation, 105064, Moscow; 119048, MoscowVitaly V. Zverev
I. Mechnikov Research Institute of Vaccines and Sera; Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University)
Email: fgn42@yandex.ru
ORCID iD: 0000-0001-5808-2246
D. Sci. (Biol.), Professor, RAS Full Member, Head of Laboratory of molecular biotechnology
Russian Federation, 105064, Moscow; 119048, MoscowReferences
- Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers. 2015; 1: 15016. https://doi.org/10.1038/nrdp.2015.16
- Arvin A.M., Moffat J.F., Abendroth A., Oliver S.L., eds. Varicella-zoster Virus. Genetics, Pathogenesis and Immunity. 6th ed. Cham: Springer; 2023. https://doi.org/10.1007/978-3-031-15305-1
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014; 89(25): 265–87.
- Heininger U., Seward J.F. Varicella. Lancet. 2006; 368(9544): 1365–76. https://doi.org/10.1016/S0140-6736(06)69561-5
- Harpaz R., Ortega-Sanchez I.R., Seward J.F. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2008; 57(RR-5): 1–30; quiz CE2-4.
- Cohen J.I. Clinical practice: Herpes zoster. N. Engl. J. Med. 2013; 369(3): 255–63. https://doi.org/10.1056/NEJMcp1302674
- Shin D., Shin Y., Kim E., Nam H., Nan H., Lee J. Immunological characteristics of MAV/06 strain of varicella-zoster virus vaccine in an animal model. BMC Immunol. 2022; 23(1): 27. https://doi.org/10.1186/s12865-022-00503-6
- Higashimoto Y., Hattori F., Kawamura Y., Kozawa K., Hamano A., Kato M., et al. Analysis of the reliability of rapid diagnostic tests for varicella, including breakthrough cases. J. Med. Virol. 2023; 95(2): e28569. https://doi.org/10.1002/jmv.28569
- Pan D., Wang W., Cheng T. Current methods for the detection of antibodies of varicella-zoster virus: a review. Microorganisms. 2023; 11(2): 519. https://doi.org/10.3390/microorganisms11020519
- Otani N., Shima M., Tanimura S., Ueda T., Ichiki K., Nakajima K., et al. Sensitivity and specificity of different antibody tests for detecting varicella-zoster virus. J. Infect. Chemother. 2020; 26(12): 1283–7. https://doi.org/10.1016/j.jiac.2020.07.012
- Mo C., Lee J., Sommer M., Grose C., Arvin A.M. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002; 304(2): 176–86. https://doi.org/10.1006/viro.2002.1556
- Berarducci B., Rajamani J., Reichelt M., Sommer M., Zerboni L., Arvin A.M. Deletion of the first cysteine-rich region of the varicella-zoster virus glycoprotein E ectodomain abolishes the gE and gI interaction and differentially affects cell-cell spread and viral entry. J. Virol. 2009; 83(1): 228–40. https://doi.org/10.1128/JVI.00913-08
- Hwang J.Y., Kim Y., Lee K.M., Shin O.S., Gim J.A., Shin Y., et al. Cross-reactive humoral immunity of clade 2 Oka and MAV/06 strain-based varicella vaccines against different clades of varicella-zoster virus. Hum. Vaccin. Immunother. 2023; 19(1): 2210961. https://doi.org/10.1080/21645515.2023.2210961
- Marin M., Güris D., Chaves S.S., Schmid S., Seward J.F. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007; 56(RR-4): 1–40.
- Сenters for Disease Control and Prevention. Chickenpox (Varicella). Available at: https://cdc.gov/chickenpox/hcp/index.html
- Lafreniere M.A., Badr E., Beattie J., Macri J., Khan W.I. Performance evaluation system of the Bio–Rad Bioplex 2200 multiplex system in the detection of measles, mumps, rubella, and varicella-zoster antibodies. J. Clin. Virol. Plus. 2023; 3(1): 100131. https://doi.org/10.1016/j.jcvp.2022.100131and
- Coates S.R., Madsen R.D., Rippe D.F. New passive hemagglutination assay kit that uses hemagglutinin-sensitized erythrocytes for detection of rubella antibodies. J. Clin. Microbiol. 1982; 16(6): 1117–22. https://doi.org/10.1128/jcm.16.6.1117-1122.1982
- Kim K.S., Sapienza V., Chen C.M. Confirmation of human cytomegalovirus by reverse passive hemagglutination with monoclonal antibodies reactive to the major glycosylated peptide (GP-66). J. Clin. Microbiol. 1986; 24(3): 474–7. https://doi.org/10.1128/jcm.24.3.474-477.1986
- Maduike C.O., Ezeibe A.A., Anene N.I., Amechi B., Eze J.I., Animoke P.C. Direct passive hemagglutination test for rapid quantification of plasma load of the human immunodeficiency virus. Sci. Res. 2013; 5(9): 1351–4. http://dx.doi.org/10.4236/health.2013.59183
- Wasmuth E.H., Miller W.J. Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J. Med. Virol. 1990; 32(3): 189–93. https://doi.org/10.1002/jmv.1890320310
- Kino Y., Minamishima Y. Passive hemagglutination assays for the detection of antibodies to herpes viruses. Microbiol. Immunol. 1993; 37(5): 365–8. https://doi.org/10.1111/j.1348-0421.1993.tb03223.x
- Weinbach R. Die Verwendbarkeit formolbehandelter Erythocyten als Antigtntrager in der Haemagglutination. Schweiz. Z. Pathol. Bakteriol. 1958; 21(6): 1043–52. https://doi.org/10.1159/000160565 (in German)
- Frigo N.V., Komarova V.D., Obryadina A.P., Burkov A.N. Comparative results of enzyme immunoassay, reactions of passive hemagglutination and microreaction in serodiagnostics of syphilis. Vestnik dermatologii i venerologii. 2000; (4): 4–36. (in Russian)
- Mendelson E., Aboudy Y., Smetana Z., Tepperberg M., Grossman Z. Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV). Reprod. Toxicol. 2006; 21(4): 350–82. https://doi.org/10.1016/j.reprotox.2006.02.001
- Nagieva F.G., Barkova E.P., Lisakov A.N., Sidorov A.V., Zverev V.V., Osokina O.V., et al. Practical aspects on identification, cultivation and characteristics of varicella-zoster virus isolates. Infektsiya i immunitet. 2020; 10(2): 387–96. https://doi.org/10.15789/2220-7619-PAO-1211 https://elibrary.ru/ptnvte (in Russian)
- Shubladze A.K., Gaidamovich S.Ya. Short Course of Practical Virology [Kratkii kurs prakticheskoi virusologii]. Moscow: Medgiz; 1954. (in Russian)
Supplementary files