Molecular Study of Varicella zoster virus in Cerebrospinal Fluid from Stroke Patients of Thi-Qar province
- Authors: Salim Z.F.1,2, Hamad B.J.1
-
Affiliations:
- University of Thi-Qar
- Ministry of Health, Thi-Qar Health Directorate
- Issue: Vol 69, No 4 (2024)
- Pages: 341-348
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16647
- DOI: https://doi.org/10.36233/0507-4088-245
- EDN: https://elibrary.ru/ovtgzm
- ID: 16647
Cite item
Full Text
Abstract
Introduction: Varicella zoster virus (VZV) is a type of alpha-herpesvirus that specifically targets the nervous system. The initial infection, typically occurring during childhood, results in varicella (commonly known as chickenpox), after which the virus enters a dormant state in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia throughout the entire neuroaxis.
Aim of the study: Molecular and genetic studies of viruses are an important tool for virus development and identifying viral treatments to combat the diseases. The aim of the study was to determine the whole ORF4 sequence of the local VZV strains for phylogenetic analysis to determine the variability in the viral sequence.
Material and methods: Ten samples of VZV DNA were subjected to the sequencing of the whole ORF4 region following identification using the PCR method.
Results: Sequences from five samples have been successfully analyzed. All clinical strains were discovered to possess a genome with a length of 124,884 base pairs. The sequences exhibited the occurrence of two distinct mutations, one being a transversion and the other a transition, with the latter resulting in an alteration of the amino acid. A phylogenetic tree was constructed using the maximum likelihood method based on the sequences of five nucleotide sequences from clinical samples and nine reference VZV strains. The tree displayed the evolutionary distances between these sequences. The analysis of the phylogenetic tree revealed the presence of five primary clades, with four of them originating from India (isolates S1, S2, S4, S5), while S3 exhibited similarity to a strain from the United Kingdom.
Keywords
Full Text
Introduction
Varicella-Zoster virus (VZV) is a pathogenic human herpesvirus that causes varicella (chicken pox) as a primary infection [1]. VZV, which was latent in the cranial nerve or dorsal root ganglia, reactivates to cause the viral shingle. VZV can reactivate due to immunosenescence which includes the known decrease in cell-mediated immunity with age [2]. It continues to be acknowledged as a significant global public health concern that impacts individuals across several geographic regions. Typically, 10–20% of people over 50 have experienced at least one zoster episode in their entire life [3]. The neurotropic human alpha herpesvirus VZV is endemic to every country in the world. It creates a permanent latency in neurons after initial infection, and periodically reactivates to cause a range of moderate to severe illnesses [4]. Studies of genetic diversity of VZV play an important role in further understanding of the epidemiology and evolution of the virus and may in future serve as a tool for genetic prediction of virus pathogenicity or resistance development [5].
The cerebrospinal fluid (CSF) of individuals with neurological consequences from VZV infection has a high concentration of chemokines and an inflammatory response, which attract immune cells and inflame central nervous system (CNS) tissues [6]. Reactivation of the VZV has been associated with a higher risk of stroke [7]. An infection with the VZV may result in vascular inflammatory alterations that raise the risk of stroke [8]. The VZV, which is a member of the subfamily Alphaherpesvirinae family within the Herpesviridae family, replicates and causes illness in the arteries of the human brain [9]. When VZV infects the cerebral arteries, it causes vasculopathy, which can lead to stroke. VZV encephalitis usually develops following primary infection and reactivation. Vasculopathy can also develop due to the primary infection or VZV reactivation [10]. According to statistics from the World Health Organization (WHO) in 2014, complications from VZV infection resulted in over 4 million hospital admissions and over 4,000 fatalities worldwide annually. The intraocular distribution of the trigeminal nerve harbors VZV, which is associated with a 4.5-fold higher risk of stroke [11].
Worldwide, one in five persons may encounter VZV at some point in their lives, and around one-third of those who do will go on to develop herpes zoster (HZ) [12]. Commonly used techniques for diagnosing VZV infection include advanced pathogen testing as well as conventional medical exams. CSF testing, standard blood testing and biochemical testing are among the common medical procedures [13].
Materials and methods
Patients and clinical specimens
In total, 120 samples were obtained from 90 symptomatic patients with the age between 1 year and 70 years, and 30 individuals were recruited as control group. The samples were obtained from a dermatological consultant at Al-Nasiriyah Teaching Hospital and clinical private between September 2023 and January 2024. From each patient, 3 mL of CSF sample was collected under sterile conditions and put in an EDTA tube, the samples which were stored frozen at −80 °C were left to adjust to the room temperature after complete thawing.
The study was conducted with the informed consent of the patients. The research protocol was approved by the Ethics Committee of the University of Thi-Qar (Protocol No. 3/11/1260 dated 21/9/2023).
Nucleic Acid Extraction
A total of 60 μL of extracted material was recovered from 120 μL of clinical specimen using the FAVORGEN-TIWAN extraction method, following the manufacturer’s instructions. The nucleic acid was subjected to real-time PCR and melting curve analysis.
Real-Time PCR and Melting Curve Analysis
The gene targets as well as the primers used for amplification are provided in table 1. Light cycler probe design software 2.0 (Roche, Penzberg, Germany) was used to design primers except for two for internal control. The reaction mixture of total volume 20 μL included 3.0 μL of isolated nucleic acid, and one Light Cycler FastStart DNA Master SYBR Green I (Roche), 3 mM MgCl2, 0.3 M of HERV-3 primer, and each of the detection primers (1.0 μM of primers for VZV). The reaction mixture was first denatured for 10 min at 95 °C. After that, the PCR was run in Light Cycler 2.0 (Roche) for 45 cycles for 5 sec at 95 °C, 3 sec at 65 °C, and 10 sec at 72 °C [14].
Primers
The primers that were used for nucleic acid amplification are listed in the Table 1.
Table 1. Characteristics of primer used for nucleic acid amplification
Таблица 1. Характеристики праймеров, используемых для амплификации нуклеиновых кислот
Virus Вирус | Gene target Ген-мишень | Primer sequences (5'→3') Последовательности праймеров (5'→3') | Amplicon (bp) Ампликон (bp) | Reference Источник |
VZV Ветряная оспа | ORF4 | Forward primer / Прямой праймер: GCCCATGAATCACCCTC Reverse primer / Обратный праймер: ACTCGGTACGCCATTTAG | 79 | [15] |
DNA Sequencing
For constructing a phylogenetic tree and identifying the subtypes of VZV-positive samples, the ORF4 region was amplified using forward and reverse primers by PCR. The PCR products of VZV positive samples, with each tube labeled and containing 10 μl of forward and reverse primer (Table 1), were shipped to Macrogen Company in South Korea for sequencing. A phylogenetic tree was constructed using the maximum likelihood method based on the sequences of five nucleotide sequences from clinical samples and nine reference VZV strains.
Results
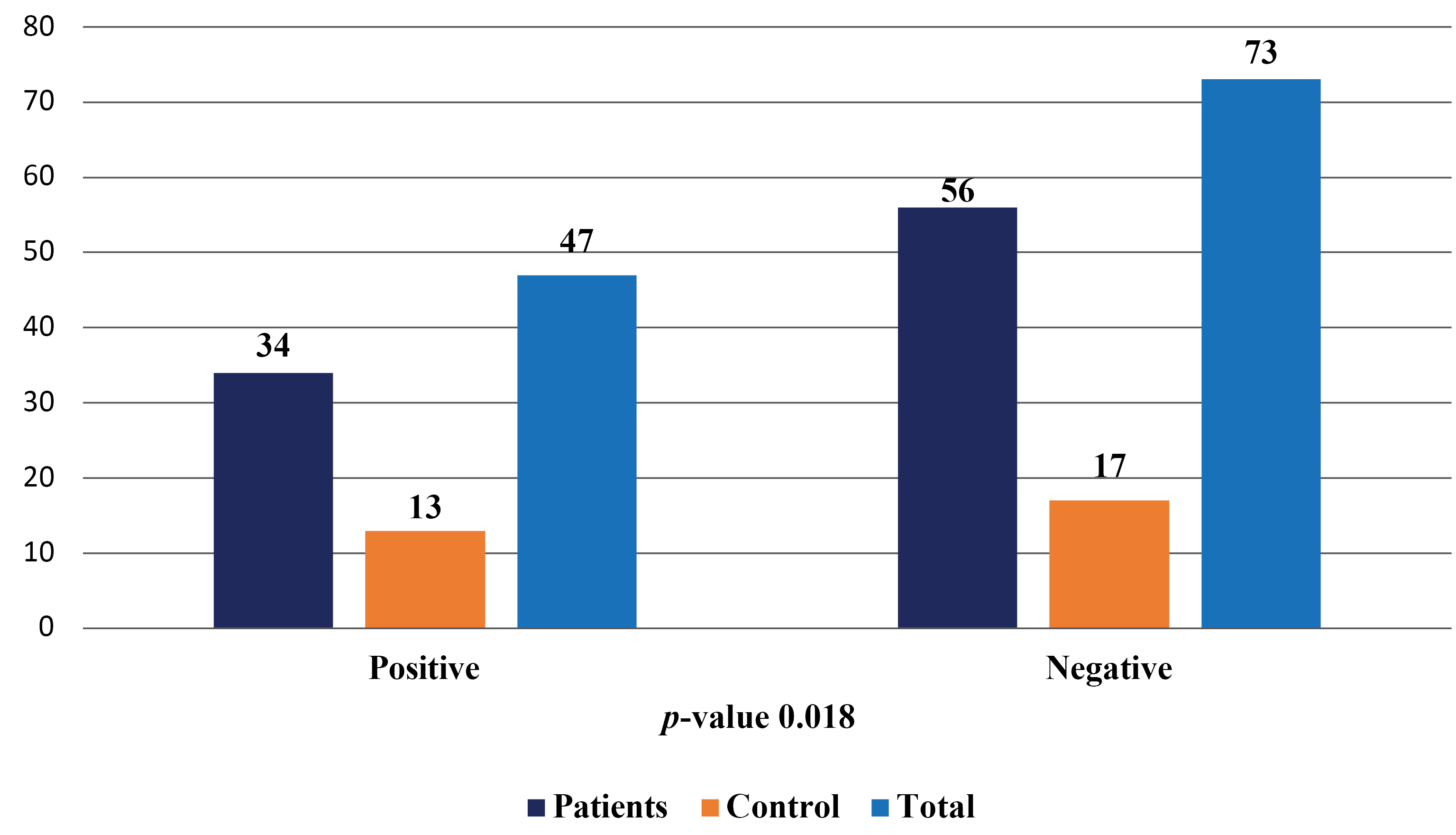
A total of 120 samples of cerebral spinal fluid were collected for this study, with 90 samples from patients with stroke and 30 samples from the control group. The study found that 47 (39.17%) of the study population were infected with VZV, with 34 (37.78%) in the patient group and 13 (43.33%) in the control group. The remaining 73 (60.83%) individuals were not infected with the virus, with 56 (62.22%) in the patient group and 17 (56.67%) in the control group. The study also observed a significant difference in the frequency of the virus in the study population, with a p-value of less than 0.05, as shown in Figure 1 and 2.
Fig. 1. Prevalence of VZV infection in control group.
Рис. 1. Распространенность VZV-инфекции в контрольной группе.
Fig. 2. Prevalence of VZV infection in stroke patients and in control group.
Рис. 2. Распространенность VZV-инфекции у пациентов с инсультом и в контрольной группе.
Mutation Detection in VZV Sequence
When comparing the sequence of isolates of the current study with several sequences deposited in the GenBank, it was found that there are two types of point mutations compared with some isolates. The first type was a transversion mutation at positions 8, 11 and 57, while the second type was a transition mutation at positions 58, 60, 61, 354 as shown in Table 2.
Table 2. Types and positions of mutations detected in VZV sequences
Таблица 2. Типы и положение мутаций, выявленных в последовательностях VZV
Isolate Изолят | Substitution Type Тип замены | Location Положение | Nucleotide Нуклеотид | Sequence ID Последовательность | Source Источник | Similarity Сходство |
1. | Transversion / Трансверсия Transition Переход | 11 354 | T/G A/G | OQ835722.1 | HAHV | 99% India 99% Индия |
2. | Transversion / Трансверсия Transition / Переход | 57 60 | C/G G/A | OQ835722.1 | HAHV | 99% India 99% Индия |
3. | Transversion / Трансверсия Transition / Переход Transition / Переход | 8 58 61 | G/T C/T G/A | KP771921.1 | HHV | 98% UK 98% Великобритания |
4. | NONE | ‒ | ‒ | OQ835722.1 | HAHV | 100% India 100% Индия |
5. | Transition / Переход Transition / Переход | 58 61 | C/T G/A | OQ835722.1 | HAHV | 99% India 99% Индия |
Phylogenetic Tree
The phylogenetic trees of the viral sequences isolated in the current study was built according to the amino acid sequence matching with the sequences deposited in NCBI. The study showed that three isolates contained genetic mutations, while two isolates were 100% identical, as in Figures 3‒7.
Fig. 3. Phylogenetic tree for the first isolate which has the level of divergence with reference sequences of 0.003.
Рис. 3. Филогенетическое дерево для 1-го изолята, у которого уровень различий с референсными последовательностями составил 0,003.
Fig. 4. Phylogenetic tree of the second isolate, which has the level of divergence with reference sequences of 0.001.
Рис. 4. Филогенетическое дерево для 2-го изолята, у которого уровень различий с референсными последовательностями составил 0,001.
Fig. 5. Phylogenetic tree of the third isolate, which has the level of divergence with reference sequences of 0.001.
Рис. 5. Филогенетическое дерево для 3-го изолята, у которого уровень различий с референсными последовательностями составил 0,001.
Fig. 6. Phylogenetic tree of the fourth isolate, which had 100% identity with reference sequences.
Рис. 6. Филогенетическое дерево для 4-го изолята, на 100% идентичного референсным последовательностям.
Fig. 7. Phylogenetic tree of the fifth isolate, isolatewhich had 100% identity with reference sequences.
Рис. 7. Филогенетическое дерево для 5-го изолята, на 100% идентичного референсным последовательностям.
Discussion
VZV belongs to the subfamily Alphaherpesvirinae. Genetically, it is divided into several genotypes based upon the genetic variations [16]. VZV is highly infectious, causes large outbreaks of varicella in the populations, and establishes lifelong latency; the subclinical reactivation of VZV, however, is believed to occur less frequently than the reactivation of other alpha herpesviruses [17]. The results of the current study are in agreement with study of Helmuth et al. [18] conducted in Denmark, that detected VSV in 9 (60%) of 15 children with arterial ischemic stroke. Also, our results are in agreement with data from Tung et al. [19] in China, who recorded the prevalence of VZV in stroke patients as high as 12.18% per 1000 patients compared to 3.63 per 1000 person per year in controls. In addition, Schmidt et al. [20] showed that mood disorders, including depression, were associated with increased risk of HZ in stroke patients. However, our data differed from study of Sundström et al. [21], in Switzerland, who reported 20.6% VZV detection rate among 112 patients with stroke. Meanwhile, this study demonstrated 6.2% prevalence in control population, similar to data from the current study. The increased detection rates of the virus in stroke patients may be due to inflammatory reactions releasing inhibitory molecules within the immune system, which are usually self-limiting and self-resolving. The release of pro-inflammatory cytokines in the brain stimulates the expansion of the immunosuppressive cell population that suppresses innate and adaptive immune responses [22]. Paradoxically, an excessive inflammatory response in the brain leads to an immunosuppressive state in peripheral tissues. Therefore, stroke patients are at risk of fatal secondary infections [23]. The study of Liu et al. [24] identified 38 polymorphic markers in four ORFs (ORFs 1, 21, 22, and 54) among 19 VZV isolates from different clinical samples in China, with considerable homology between 19 clinical strains. The previous study suggested that the ORF4 is necessary for virus replication in cell culture. Furthermore, this gene is essential for the establishment of latency, but it is not necessary for latent infection. As a result, the researchers deleted the ORF4 from the viral genome and were unable to produce an infectious virus. However, when they inserted the ORF4 into a cosmid at a nonnative site, they obtained virus. The results of the current study are consistent with the previous study by Sato et al. [25], who observed 13 positive samples in the control group and 34 in stroke patients, and suggested that ORF4 was required for replication and latency, but not not required to induce acute infection. Recent studies in animal experiments have demonstrated based on the data from transcriptional analyzes of the ICP27 protein expressed by ORF4, that neither HSV infection nor global ICP27 overexpression inhibits splicing [26], but rather modulates alternative splicing of a subset of cells [27]. HSV virus causes host-induced shutdown, and another study suggested that exposure of the virus to deletion and substitution mutations in the ORF4 proteins may enable the virus to activate the binding mechanism and cause a latency state in the infected cell [28]. The ability of alpha herpesviruses to persist in sensory ganglia, their short reproductive cycle, their rapid dissemination, and their ability to eliminate infected cells effectively are four of their defining characteristics. VZV replication occurs primarily in cells of human and simian origin. The VZV genome has 73 genes, of which 70 are unique and three are duplicated [29]. The viral genome consists of two primary coding regions: unique long (UL) and unique short (US), as well as flanking inverted repeats known as terminal and internal repeats long (TRL, IRL) and short (TRS, IRS).
When the VZV sequences from present study were compared to sequences deposited in the GenBank, two types of point mutations were discovered. The first type was a transversion mutation at positions 8, 11 and 57, while the second type was a transition mutation at positions 58, 60, 61, and 354, as shown in Table 2, which represents the matching of the nucleotide sequences of the isolates in this study with the isolates in the database. In the isolate 1, the present study found a transversion mutation at position 11, where the purine base G was replaced with the pyrimidine base T, and other type found a mutation of the transition type at position 354, where the purine base A was replaced with the purine base G, and the strain was identical to the strain found in India. In isolate 2, the present study found a transversion mutation at position 57, where the pyrimidine base C was replaced with the purine base G, and other type found a mutation of the transition type at position 60, where the purine base G was replaced with the purine base A, and the strain was identical to the strain found in India. The current study identified three mutations in isolate 3 that corresponded to a strain of VZV found in the UK. A transversion mutation was observed at position 8, where the purine base G was replaced with the pyrimidine base T. Two transition mutations occurred at positions 58 and 61. The first involved the replacement of the pyrimidine base C with the pyrimidine base T, while the second involved the substitution of the purine base G with A. The fourth isolate, labeled as 4, did not exhibit any mutations and was found to be genetically identical to the Indian strain. In isolate 5, two mutations occurred - the same type transition in position 58 where the pyrimidine base C was replaced with the pyrimidine base T and at position 61 when the purine base G took place A, the strain matched with Indian strain. In Basrah city a comprehensive genome study revealed a resemblance to the E genotype seen in nations with a history of European colonization. The same study found the sequences contained a variety of mutations or SNPs, some of which were silent, and others produced amino acid changes [5]. VZV genotype B was detected in an isolated strain in Najaf province. No significant correlation was found between VZV genotype and life expectancy [30]. The present study demonstrated that nearly 40% of people in Thi-Qar province have been infected with VZV gene type of ORF4 in all age groups, also in fact, this gene was not actually linked to stoke infections. The emergence of the two strains in the region may be due to the frequent movement between countries in terms of travel and trade, especially since the virus is transmitted quickly through the respiratory tract between sick and healthy people.
Conclusions
The study found that about 40 percent of people in Thi-Qar had a previous infection with VZV. No significant association was observed between the occurrence of a stroke and the presence of the studied ORF4 gene of VZV in affected patients. Furthermore, two strains that were identified in the study belonged to a European clade and an Asian clade, respectively. In addition, two types of mutations, transversion and transition, occur in the viral gene ORF4.
Funding. This study was not supported by any external sources of funding.
Acknowledgement. Thanks, and appreciation to Al-Nasiriyah Teaching Hospital for granting me approvals and completing this research.
Conflict of interest. The authors declare no apparent or potential conflicts of interest related to the publication of this article.
Ethics approval. The study was conducted with the informed consent of the patients. The research protocol was approved by the Ethics Committee of the University of Thi-Qar (Protocol No. 3/11/1260 dated 21/9/2023).
About the authors
Zinah Fadhil Salim
University of Thi-Qar; Ministry of Health, Thi-Qar Health Directorate
Author for correspondence.
Email: Zeena.salim.ph@utq.edu.iq
ORCID iD: 0009-0007-5565-502X
Master Student, lecturer of Department of Biology, College of Science
Iraq, Thi-Qar, 64001; NasiriyahBushra Jabbar Hamad
University of Thi-Qar
Email: bushra.jh.bio@sci.utq.edu.iq
ORCID iD: 0000-0002-5129-7700
Assistant Professor, lecturer of Department of Biology, College of Science
Russian Federation, Thi-Qar, 64001References
- Kennedy P.G., Montague P. Variable gene expression in human ganglia latently infected with varicella-zoster virus. Viruses. 2022; 14(6): 1250. https://doi.org/10.3390/v14061250
- Rashad I. Estimation of cytokine level IL-4, IL-12, IL-17 and some immune features among patients with Herpes zoster in Thi-Qar Province/Southern Iraq. Univ. Thi-Qar J. Sci. 2023; 10(1): 87–90. https://doi.org/10.32792/utq/utjsci/v10i1.943
- Nasser H.A., Shallal M.J., Naif A., Kadhim K.A. Detection of shingles and correlation with gender, weather and residency. Biochem. Cell. Arch. 2022; 22(1): 835.
- Heinz J.L., Swagemakers S.M., von Hofsten J., Helleberg M., Thomsen M.M., De Keukeleere K., et al. Whole exome sequencing of patients with varicella-zoster virus and herpes simplex virus induced acute retinal necrosis reveals rare disease-associated genetic variants. Front. Mol. Neurosci. 2023; 16: 1253040. https://doi.org/10.3389/fnmol.2023.1253040
- Almarjan M., Al-Hmudi H.A, Habib H.N. Holegenome sequences of local varicella-zoster virus (VZV) strains of Basrah city/Iraq. Turk. J. Physiother. Rehabil. 2021; 32(3): 16094–103.
- Pormohammad A., Goudarzi H., Eslami G., Falah F., Taheri F., Ghadiri N., et al. Epidemiology of herpes simplex and varicella zoster virus in cerebrospinal fluid of patients suffering from meningitis in Iran. New Microbes New Infect. 2020; 36: 100688. https://doi.org/10.1016/j.nmni.2020.100688
- Marais G., Naidoo M., McMullen K., Stanley A., Bryer A., van der Westhuizen D., et al. Varicella-zoster virus reactivation is frequently detected in HIV-infected individuals presenting with stroke. J. Med. Virol. 2022; 94(6): 2675–83. https://doi.org/10.1002/jmv.27651
- Lu P., Cui L., Zhang X. Stroke risk after varicella-zoster virus infection: a systematic review and meta-analysis. J. Neurovirol. 2023; 29(4): 449–59. https://doi.org/10.1007/s13365-023-01144-0
- Kuriakose D., Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int. J. Mol. Sci. 2020; 21(20): 7609. https://doi.org/10.3390/ijms21207609
- Kawada J.I. Neurological disorders associated with human alphaherpesviruses. Adv. Exp. Med. Biol. 2018; 1045: 85–102. https://doi.org/10.1007/978-981-10-7230-7_5
- Wang T., Shen H., Deng H., Pan H., He Q., Ni H., et al. Quantitative proteomic analysis of human plasma using tandem mass tags to identify novel biomarkers for herpes zoster. J. Proteomics. 2020; 225: 103879. https://doi.org/10.1016/j.jprot.2020.103879
- Grahn A., Studahl M. Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment. J. Infect. 2015; 71(3): 281–93. https://doi.org/10.1016/j.jinf.2015.06.004
- Stránská R., Schuurman R., de Vos M., van Loon A.M. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J. Clin. Virol. 2004; 30(1): 39–44. https://doi.org/10.1016/j.jcv.2003.08.006
- Hieran A., Albadry B.J. A Human CCL3L1 gene expression in blood donors infected with HIV-1. Univ. Thi-Qar J. Sci. 2023; 10(1): 181–4. https://doi.org/10.32792/utq/utjsci/v10i1.1060
- Hong Y.J., Lim M.S., Hwang S.M., Kim T.S., Park K.U., Song J., et al. Detection of herpes simplex and varicella-zoster virus in clinical specimens by multiplex real-time PCR and melting curve analysis. Biomed. Res. Int. 2014; 2014: 261947. https://doi.org/10.1155/2014/261947
- Peters G.A., Tyler S.D., Grose C., Severini A., Gray M.J., Upton C., et al. A full-genome phylogenetic analysis of varicella-zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 2006; 80(19): 9850–60. https://doi.org/10.1128/jvi.00715-06
- Loparev V.N., Gonzalez A., Deleon-Carnes M., Tipples G., Fickenscher H., Torfason E.G., et al. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J. Virol. 2004; 78(15): 8349–58. https://doi.org/10.1128/jvi.78.15.8349-8358.2004
- Helmuth I.G., Mølbak K., Uldall P.V., Poulsen A. Post-varicella Arterial Ischemic Stroke in Denmark 2010 to 2016. Pediatr. Neurol. 2018; 80: 42–50. https://doi.org/10.1016/j.pediatrneurol.2017.11.018
- Tung Y.C., Tu H.P., Wu M.K., Kuo K.L., Su Y.F., Lu Y.Y., et al. Higher risk of herpes zoster in stroke patients. PLoS One. 2020; 15(2): e0228409. https://doi.org/10.1371/journal.pone.0228409
- Schmidt S.A.J., Langan S.M., Pedersen H.S., Schønheyder H.C., Thomas S.L., Smeeth L., et al. Mood disorders and risk of herpes zoster in 2 population-based case-control studies in Denmark and the United Kingdom. Am. J. Epidemiol. 2018; 187(5): 1019–28. https://doi.org/10.1093/aje/kwx338
- Sundström K., Weibull C.E., Söderberg-Löfdal K., Bergström T., Sparén P., Arnheim-Dahlström L. Incidence of herpes zoster and associated events including stroke – a population-based cohort study. BMC Infect. Dis. 2015; 15: 488. https://doi.org/10.1186/s12879-015-1170-y
- Jih J.S., Chen Y.J., Lin M.W., Chen Y.C., Chen T.J., Huang Y.L., et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm. Venereol. 2009; 89(6): 612–6. https://doi.org/10.2340/00015555-0729
- Liesz A., Dalpke A., Mracsko E., Antoine D.J., Roth S., Zhou W., et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J. Neurosci. 2015; 35(2): 583–98. https://doi.org/10.1523/jneurosci.2439-14.2015
- Liu J., Wang M., Gan L., Yang S., Chen J. Genotyping of clinical varicella-zoster virus isolates collected in China. J. Clin. Microbiol. 2009; 47(5): 1418–23. https://doi.org/10.1128/jcm.01806-08
- Sato B., Sommer M., Ito H., Arvin A.M. Requirement of varicella-zoster virus immediate-early 4 protein for viral replication. J. Virol. 2003; 77(22): 12369–72. https://doi.org/10.1128/jvi.77.22.12369-12372.2003
- Tang S., Patel A., Krause P.R. Herpes simplex virus ICP27 regulates alternative pre-mRNA polyadenylation and splicing in a sequence-dependent manner. Proc. Natl Acad. Sci. USA. 2016; 113(43): 12256–61. https://doi.org/10.1073/pnas.1609695113
- Rutkowski A.J., Erhard F., L’Hernault A., Bonfert T., Schilhabel M., Crump C., et al. Widespread disruption of host transcription termination in HSV-1 infection. Nat. Commun. 2015; 6: 7126. https://doi.org/10.1038/ncomms8126
- Kennedy P.G.E., Mogensen T.H., Cohrs R.J. Recent issues in varicella-zoster virus latency. Viruses. 2021; 13(10): 2018. https://doi.org/10.3390/v13102018
- Davison A.J. In: Arvin A.M., Gershon A.A., ed. Varicella-Zoster Virus. Cambridge: Cambridge University Press; 2000; 25–50. https://doi.org/10.1017/CBO9780511601194
- Al-Khafaji Z.A., Mahmood T.A., Alhuchaimi S.N. Molecular study of varicella-zoster virus infection among individuals in Najaf province/Iraq. Int. J. ChemTech Res. 2017; 10(2): 683–91.
Supplementary files