Epstein–Barr virus (Orthoherpesviridae: Lymphocryptovirus) among Russian ethnic groups: Prevalence of EBV types (EBV-1 and EBV-2), LMP1 gene variants and malignancies
- Authors: Gurtsevitch V.E.1, Lubenskaya A.K.1, Senyuta N.B.1, Smirnova K.V.1,2,3
-
Affiliations:
- Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
- The Peoples’ Friendship University of Russia (RUDN University)
- Pirogov Russian National Research Medical University (Pirogov Medical University)
- Issue: Vol 69, No 1 (2024)
- Pages: 56-64
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16609
- DOI: https://doi.org/10.36233/0507-4088-214
- EDN: https://elibrary.ru/fibzll
- ID: 16609
Cite item
Abstract
Introduction. The discovery of two EBV types (EBV-1 and EBV-2) has stimulated the study of their prevalence in populations and association with malignancies.
Objective. To study the prevalence of EBV-1 and EBV-2 types among ethnic groups in Russia, to analyze PCR products of the LMP1 gene in virus isolates, and to evaluate the contribution of EBV types to the incidence of malignant neoplasms.
Materials and methods. EBV isolates from oral lavages of the Republics Adygea, Kalmykia, Tatarstan and the Moscow Region (MR) representatives were studied by nested PCR for the belonging to EBV-1 and EBV-2 types. LMP1 amplicons obtained by real-time PCR from viral isolates DNA were classified and sequenced on an automatic DNA sequencer ABI PRISM 3100-Avant (USA). The sequencing results were analyzed using Chromas 230 and Vector NT programs (Invitrogen, USA). The reliability of the obtained data was assessed using statistical packages Statistica for Windows, 10.0.
Results. The prevalence rates of EBV-1 and EBV-2 in representatives of four ethnic groups were compared with the incidence rates of some tumors in the population of three Republics and MR.
The dominant persistence of the transforming in vitro EBV-1 type in representatives of the Republic of Tatarstan and MR correlated with a high incidence of gastric cancer and lymphomas in the population of these territories. On the contrary, predominant infection of the non-transforming in vitro EBV-2 type and both types of the virus in approximately the same percentage of representatives of Adygea and Kalmykia, respectively, correlated with a lower level incidence of above tumors in populations of these Republics. The differences between the incidence rates of neoplasms in the compared ethnic populations were statistically insignificant (p > 0.05). LMP1 variants of viral isolates did not reflect either the level of EBV persistence types or the incidence of tumors.
Conclusion. Infection of ethnic groups with EBV-1 and EBV-2 may vary significantly under the influence of various factors. The predominance of the in vitro transforming EBV-1 type in the population did not increase the incidence of tumors due to cases associated with the dominant virus type.
Full Text
Introduction
Epidemiological data suggest that the Epstein-Barr virus (EBV) is involved in the occurrence of approximately 200 thousand tumors per year worldwide [1]. Moreover, the biological peculiarity of EBV lies in its ability to induce neoplasia of various cellular origins, while tumors caused by other oncogenic viruses arise only in the cells of their target tissues. On the other hand, it is known that EBV infects more than 90% of the world’s population, but its permanent presence in the human body is not a prerequisite for the occurrence of a tumor. To realize the oncogenic potential of the virus, the participation of additional factors, both common and different for different types of tumors, is necessary. In particular, it has been proven that the risk of developing endemic Burkitt’s lymphoma in the countries of Equatorial Africa is associated with a high burden of EBV infection in children at birth, coupled with Plasmodium falciparum infection, which is mutagenic on B cells. [2]. The risk of developing nasopharyngeal carcinoma (NPC) is also contributed by high infection with EBV in infancy and dietary habits from the first years of a child’s life, characteristic of the Southern China culture – feeding children salted fish, rich in precarcinogens (nitrosamines) [3]. Impaired immunity, changing the balance between the virus and the host in favor of the virus, is also an important prerequisite for the occurrence of both types of tumors.
In the occurrence of some tumors, for example, NPC, the genetic element also plays an important role. So-called familial cancer cases account for approximately 10% of all NPC cases reported for the population in the NPC-endemic southern provinces of China. [4]. Several allelic susceptibility determinants most closely associated with the HLA class I region have also been found in patients with NPC outside of endemic regions [5].
With the discovery of two EBV types, EBV-1 and EBV-2, differing in the genes encoding nuclear antigens (EBNA-2, -3A, -3B, -3C) and biological properties, investigations began to be carried out aimed at elucidation the role of each type in EBV-associated carcinogenesis. These studies were based on the fact, that EBV-1, unlike EBV-2, is able to transform B cells in vitro [6] and on the results of experimental studies showing slower growth of lymphoblastoid cell lines infected with EBV-2, compared to those with EBV-1. It turned out, however, that both types of the virus were capable of causing B-cell lymphomas in CBH mice [7].
Both types of virus also differ in the degree of prevalence among different populations. In particular, it has been shown that the Caucasian population is more often infected with type 1 virus (~74%), and its even greater predominance is observed in healthy individuals of Asian origin (~85%) [8, 9]. There are also data indicating a fairly wide spread of EBV-2 among certain population groups, for example, in persons asymptomatically infected with HIV (50%) [10], patients with progressive form of HIV infection (62%), AIDS patients with non-Hodgkin’s lymphoma (53%), and even among American donors in certain US states (50%) [11]. However, the question of whether EBV-1 and EBV-2 are tumor-specific remains unanswered to this day.
The search for tumor-specific EBV strains regardless of the type of virus was also carried out based on the genetic diversity of the LMP1 genes, classified according to the generally accepted classification of Edwards et al. [12]. In particular, almost all EBV isolates of Chinese origin belonging to the virus type 1 were found to contain a 30-bp deletion with characteristic amino acid (aa) substitutions in its protein variant, LMP1 [13]. In populations from other geographic regions, such as Japan, the 30-bp deletion found in LMP1 was associated predominantly with type 2 virus. [14]. It is important to note that the majority of molecular polymorphisms found in EBV isolates from healthy virus carriers are also found with equal frequency in virus-associated patient’s tumors from in the same geographic region. [15].
The question arises whether EBV types and/or LMP1 virus variants can influence the incidence of certain forms of tumors or whether the frequency of the latter is determined mainly by the genetic characteristics of the population and/or other factors. In this regard, it would be important to study the molecular profile as well as the tumor-inducing properties of EBV isolates among ethnic groups representing genetically distinct populations and exposed to different environmental factors. Based on the above, the purpose of this study was to analyze the prevalence of EBV types and LMP1 variants in representatives of four genetically distinct ethnic groups (Adygeans, Kalmyks, Tatars and Slavs), residents of different climatic zones and geographical territories of Russia with their own lifestyle and culture. It was also important to find out whether there is a correlation between the dominance of one of the EBV types and/or LMP1 variant and the incidence of malignant tumors.
Materials and methods
Objects of research
Oral lavages from representatives of four ethnic groups – Adygeans, Kalmyks, Tatars and Slavs, residents of three Republics of the Russian Federation and the Moscow region (MR), respectively, were studied. Detailed characteristics of the individuals participating in the study are presented in Table 1. All participants were practically healthy people and representatives of the above ethnic groups no less than in three generations. Each lavage was a cell suspension obtained individually after rinsing the mouth for 30 s with 15 mL of sterile saline solution. Lavage samples collected in hermetically sealed plastic tubes were stored at a temperature of +4 °C for no more than two days before the study. Informed consent was obtained from all individuals examined. The research protocol was approved by the Ethics Committee of the N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia (Protocol No. 512 of November 10, 2021).
Table 1. Characteristics of ethnic group representatives involved in the study
Таблица 1. Характеристика представителей этносов, участвовавших в исследовании
Repsentatives of ethnic groups Представителиэтносов | Religion Религия | Geographical region Географический регион | Number of investigated persons Число обследованных лиц | Gender ratio: M/F Гендерное соотношение: М/Ж | Average age, years Средний возраст, лет |
Adygeans Адыгейцы | Islam Ислам | Maykop town, R. Adygea г. Майкоп, Р. Адыгея | 59 | 24/34 | 41,4 |
Kalmyks Калмыки | Buddhism Буддизм | Elista town, R. Kalmykia г. Элиста, Р. Калмыкия | 50 | 19/31 | 21,4 |
Tatars Татары | Islam Ислам | Kazan city, R. Tatarstan г. Казань, Р. Татарстан | 60 | 15/45 | 21,5 |
Slavs Славяне | Orthodoxy Православие | Small towns, Moscow region Московская область, районы | 40 | 21/19 | 47,5 |
DNA extraction and LMP1 gene amplification
Samples of total DNA from oral cells lavages collected after centrifugation were isolated by phenol-chloroform deproteinization. The presence and concentration of EBV DNA amplified from total DNA samples was analyzed by real-time polymerase chain reaction (PCR), which we described previously [16]. Amplification of the LMP1 gene from EBV DNA was carried out in two stages with external and internal primers according to our previously adopted method [17]. Each PCR product was purified on a QIAGEN mini-column (QIAquick PCR Purification kit, cat. 28104, Germany) according to the manufacturer’s instructions. Approximately 100-200 ng of PCR product was used for the reaction, and the DNA concentration was assessed visually in an agarose gel. DNA isolated from the B95-8 cell line was used as a positive control, and water was used as a negative control.
EBV typing with nested PCR of the EBNA-2 gene
Typing of EBV isolates into EBV-1 and EBV-2 types was carried out using nested PCR, following the previously described method [18] with minor modifications. The primers used demonstrated high specificity and lack of cross-reactivity with the human genome and other viruses or microorganisms [19].
Sequencing of LMP1 PCR products
LMP1 amplicons were sequenced in both directions. Sequencing was performed using the ABI PRISM® BigDye Terminator v. 3.1 reagent kit (USA), followed by analysis of the reaction products on an automatic DNA sequencer ABI PRISM 3100-Avant (USA). Sequencing results were processed using Chromas 230 and Vector NT programs (Invitrogene, USA).
LMP1 classification
The nucleotide LMP1 sequences amplified from EBV isolates obtained from oral lavages were translated into LMP1 amino acid sequences and analyzed using the literature-accepted classification of Edwards et al. [12].
Statistical analysis
The number of EBV-1 and EBV-2 copies in individual’s oral lavages of the study groups was assessed using the Mann–Whitney U test. Fisher’s exact test was used to calculate the p value when comparing the number of individuals infected with EBV type 1 or 2; differences were considered statistically significant at p ≤ 0.05. Calculations were carried out using statistical packages Statistica for Windows, 10.0.
Results
EBV types
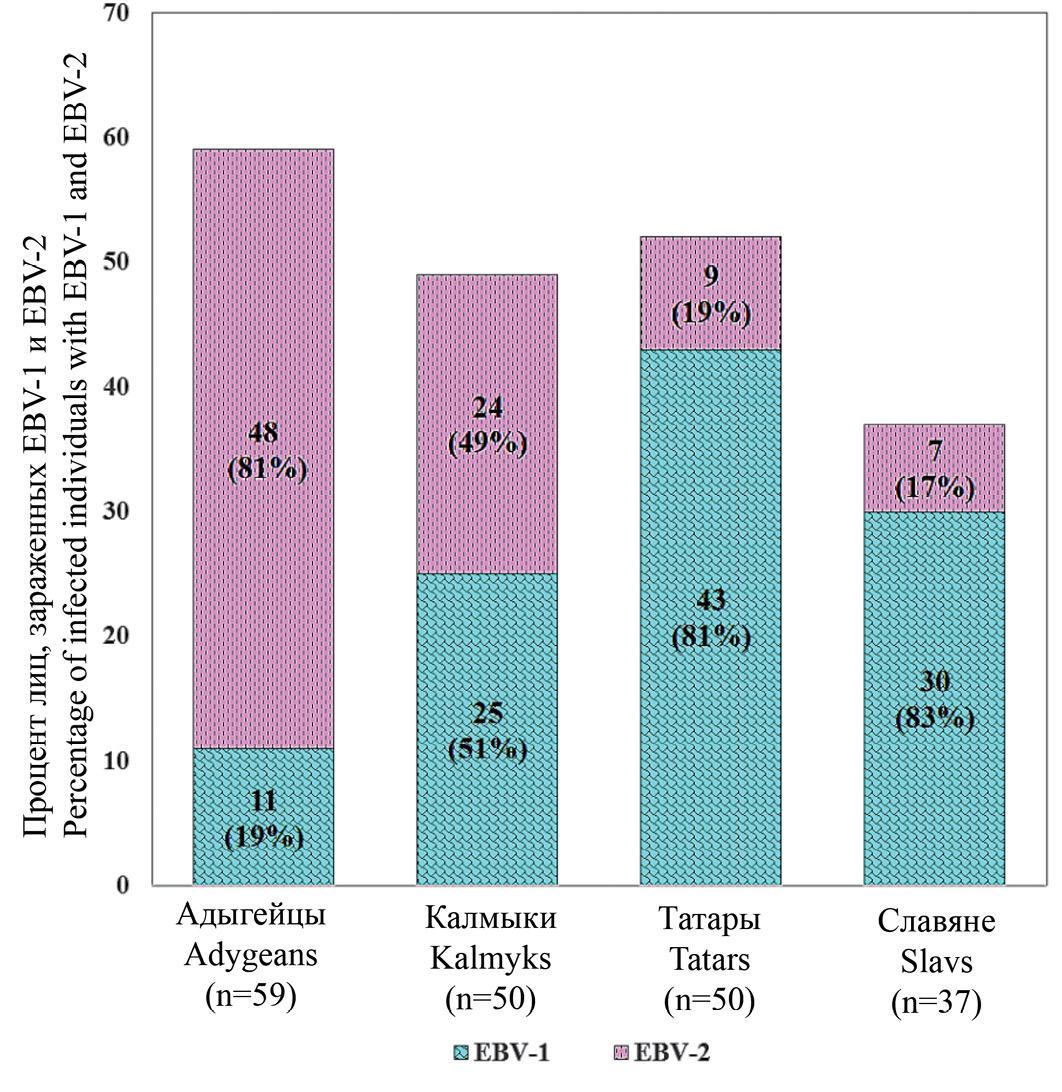
EBV samples amplified from cell suspensions of four ethnic groups representatives were tested for the EBV-1 and EBV-2 virus types. The results obtained indicate (Figure 1) that EBV-1 dominates among representatives of the Slavs and Tatars (81%, 30/37; and 83%, 43/52, respectively), and EBV-2 dominates among representatives of the Adygeans (81%; 48 /59 respectively). Representatives of the Kalmyks were infected with both types of the virus in approximately equal proportions (with EBV-1 – 51%, 25/49 and with EBV-2 – 49%, 24/49). Simultaneous infection with both types of the virus was found per one case in the groups of Kalmyks, Slavs and Tatars.
Fig. 1. The ratio of EBV-1 and EBV-2 in oral lavages of four ethnic groups’ representatives: Adygeans, Kalmyks, Tatars and Slavs.
Рис. 1. Соотношение ВЭБ-1 и ВЭБ-2 в смывах полости рта представителей четырех этносов: адыгейцев, калмыков, татар и славян.
Polymorphism of EBV LMP1 gene samples
The nucleotide sequences of the LMP1 gene samples were translated into amino acids, followed by determination of the variant for each LMP1 sample using Edwards et al. classification [12]. Detected LMP1 variants, as well as the results of their sequencing, are presented in Table 2. It follows from the table that the low transforming in vitro LMP1-95.8 variant was characteristic for 100% of Adygean’s viral isolates. This LMP1 variant was also widely presented among Kalmyk’s and Slav’s viral isolates (75.9% and 82.5%, respectively), and to a lesser extent – among Tatar’s ones (34.1%). The LMP1-China-1 variant, an analogue of the highly transforming in vitro Chinese variant LMP1-Cao, was identified among viral isolates of Kalmyks (17.2%), Slavs (7.5%), and Tatars (9.8%). The LMP1-Med- (minus) variant among viral isolates of the above ethnic groups representatives was found in 3.4%, 2.5% and 14.6% of cases, respectively), and the LMP1-NC variant was found only among representatives of Kalmyks (3.4%) and of Slavs (7.5%).
Table 2. LMP1 polymorphism in EBV isolates from oral lavages of four ethnic groups representatives
Таблица 2. Полиморфизм LMP1 в изолятах ВЭБ из смывов полости рта представителей четырех этносов
Investigated ethnic groups (number of oral lavages) Этническая группа (количество промываний полости рта) | Number of amplified LMP1 samples Число амплифицированных образцов LMP1 | LMP1 variants by classification of Edwards et al. [12] Варианты LMP1 по классификации R. Edwards и соавт. [12] | Mutations in CTAR regions of the LMP1 gene Мутации в CTAR-областях гена LMP1 | ||||||
B95.8 | China-1 | Med- | NC | ВК* OC* | CTAR 1 191-232 | CTAR 2 351-386 | CTAR 3 275-330 | ||
Adygeans Адыгейцы (n = 59) | 29/59 (49,2%) | 29/29 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | – | S229T 2/29 (6,9%) | S366T 1/29 (3,4%) | S309T/N 2/29 (6,9%) |
Kalmyks Калмыки (n = 50) | 29/50 (58,0%) | 22/29 (75,9%) | 5/29 (17,2%) | 1/29 (3,4%) | 1/29 (3,4%) | – | S229T 2/29 (6,9%) | S366A/T 6/29 (20,7%) | S309/N; 8/29 (16,9%) Q322E/T 7/29 (24,1%) |
Slavs Славяне (n = 40) | 40/40 (100%) | 33/40 (82,5%) | 3/40 (7,5%) | 1/40 (2,5%) | 3/40 (7,5%) | – | S229T 8/41 (19,5%) | S366A/T 22/41 (53,6%) | S309N; 2/41(4,9%) Q322N/E; 9/41(22,0%) |
Tatars Татары (n = 60) | 41/60 (68,3%) | 14/41 (34,1%) | 4/41 (9,8%) | 6/41 (14,6%) | 0 (0%) | 17/41 (41,5%) | 0 (0%) | S366/T 19/40 (47,5%) L338S 7/40 (17,5%) | S309N; 4/40 (10,0%) Q322N/E/T 7/40 (17,5%) E328Q; 4/12 (30,0%) |
Note. *OC – out of classification.
Примечание. *ВК – вне классификации.
Out of 41 LMP1 samples of Tatar’s representatives according to the classification of Edwards et al., 24 LMP1 variants were identified. The remaining 17 samples (41.5%) could not be interpreted using the above mentioned classification, which allowed us to designate these samples as “out of classification” (OC). Among the 17 unidentified LMP1 samples, a group of eight samples was characterized by a combined content of 5 aa deletions in codons 312–316 and 382–386, which are not characteristic of any of the LMP1 variants known to us. It is apparent that this group, related to the ethnic Tatars and designated as LMP1-TatK (Tatarstan-Kazan), deserves further investigation.
Sequencing of LMP1 samples obtained revealed the presence of important key mutations in the C-terminal region of the trans-activating domains (CTARs). In particular, in the CTAR1 domain of LMP1 samples belonging to representatives of the Adygeans, Kalmyks and Tatars, a mutation in codon 229 (S→T) was detected in 6.9%, 6.9% and 19.5% of cases, respectively. In the CTAR2 domain, LMP1 samples from representatives of all four ethnic groups contained mutations at codon 366 (S→A/T) in a range from 3.4% to 53.6% of cases. In the CTAR3 domain, LMP1 samples also obtained from representatives of all ethnic groups contained a mutation at codon 309 (S→T/N) with a frequency of 4.9% to 16.9% of cases. In the same domain, among representatives of the Kalmyks, Tatars and Slavs, the mutation (Q→N/E/T) at codon 322 was found in 24.1%, 22.0% and 17.5% of cases, respectively, and the mutation at codon 328 (E →Q) was detected only among representatives of the Slavs in 30% of cases (4/12). Based on the results of sequencing LMP1 samples from representatives of the Adygeans, Kalmyks and Tatars, we can conclude that the EBV strains circulating among these ethnic groups are genetically related. EBV strains of Slavic origin, although characterized by the absence of mutations in the CTAR1 domain and an increased number of mutations in the CTAR2 and CTAR3 domains, taking into account the other detected mutations, can also be considered genetically close to EBV strains circulating in other representatives of the studied ethnic groups.
EBV types and malignant tumors
To find out whether each type of EBV affects the incidence of malignant tumors the prevalence rates of EBV-1 and EBV-2 among representatives of the Adygeans, Kalmyks, Tatars and Slavs were compared with the incidence rates of certain malignant neoplasms among the population of three Republics and the Moscow region. The incidence rates of tumors of the stomach, pharings, oral cavity and blood were analyzed, in which corresponding EBV-associated neoplasms occur, such as gastric adenocarcinoma, tonsil cancer and nasopharyngeal carcinoma, Hodgkin’s lymphoma, and non-Hodgkin’s lymphomas (Figure 2). According to standardized values (per 100,000 population), the incidence of the oral cavity and pharynx tumors among the population of three Republics and the MR was low and ranged from 7 to 36 [20]. The incidence of gastric cancer, lymphomas were significantly higher. Their values for R. Tatarstan (121 and 95, respectively) and MR (124 and 103, respectively) correlated with the dominance of the in vitro transforming type EBV-1 in representatives of these regions. On the contrary, lower incidence rates of the same tumors were observed in the population of the R. Adygea (72 and 81, respectively), whose representatives were infected predominantly with a non-transforming virus type, EBV-2, and the population of the R. Kalmykia (63 and 50, respectively), whose representatives were infected with both types of the virus in approximately equal proportions. Statistical analysis, however, showed that the differences between the incidence rates of gastric cancer and lymphomas among the populations of the R. Tatarstan and the MR, on the one hand, and the R. Adygea and the R. Kalmykia, on the other hand, were statistically insignificant (p > 0.05).
Fig. 2. Incidence rates of malignant neoplasms with EBV-associated cases among the population of the Republics of Adygea, Kalmykia, Tatarstan and the Moscow region.
Рис. 2. Показатели заболеваемости опухолями с ВЭБ-ассоциированными случаями среди населения республик Адыгея, Калмыкия, Татарстан и Московской области.
Discussion
We studied the prevalence of EBV types, EBV-1 and EBV-2, in representatives of four ethnic groups that are genetically distinct and inhabit different geographical and climatic regions of Russia. It has been shown that the ratio of individuals infected with EBV-1 and EBV-2 among representatives of each ethnic group was different, which is likely due to the genetic characteristics of the ethnic groups and, above all, the diversity of their MHC types. Likely for the same reason, EBV-1 is dominating in the Caucasian population, while the population of certain African countries is more often infected with EBV-2 [21]. It also seems impossible to exclude the influence of climate and living conditions on the spread of virus types.
Attempts to discover the existence of tumor-specific EBV strains have been made by many researchers, but have so far been unsuccessful. In this study, the dominance of the transforming in vitro EBV-1 type among representatives of the R. Tatarstan and the MR correlated with a high incidence of gastric cancer and lymphomas in the population of these territorial entities. On the contrary, lower incidence rates of the same neoplasms among the population in other studied Republics were combined with the predominant spread of the non-transforming EBV-2 type among representatives of the R. Adygea and both types of the virus in almost equal ratio among representatives of the R. Kalmykia. However, the differences between the incidence rates of these tumors in the ethnic groups studied turned out to be statistically insignificant (р > 0.05). Nevertheless, the data obtained are of interest and require additional research. It can be assumed that in order to clarify the effect of EBV infection on the incidence of malignant tumors, it would be important to determine the frequency of EBV-associated cases among the tumors of studied location. Establishing the ratio of tumors associated with transforming and non-transforming types of EBV, EBV-1 and EBV-2 could also make an important contribution to the study of EBV-associated carcinogenesis.
About the authors
Vladimir E. Gurtsevitch
Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Author for correspondence.
Email: gurtsevitch-vlad-88@yandex.ru
ORCID iD: 0000-0003-1840-4364
Dr. Sci. (Medicine), Professor, Chief Scientific Adviser of the Laboratory of Viral Carcinogenesis, Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Russian Federation, 115478, MoscowAlexandra K. Lubenskaya
Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Email: lubenskoy.96@mail.ru
ORCID iD: 0000-0003-3953-7449
Researcher, Laboratory of Viral Carcinogenesis, Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Russian Federation, 115478, MoscowNatalia B. Senyuta
Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Email: nat.senyuta@yandex.ru
ORCID iD: 0000-0001-8915-8274
PhD (Medicine), Scientific Consultant of the Laboratory of Viral Carcinogenesis, Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia
Russian Federation, 115478, MoscowKsenia V. Smirnova
Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia; The Peoples’ Friendship University of Russia (RUDN University); Pirogov Russian National Research Medical University (Pirogov Medical University)
Email: skv.lab@yandex.ru
ORCID iD: 0000-0001-6209-977X
PhD (Biology), Head of the Laboratory of Viral Carcinogenesis, Research Institute of Carcinogenesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia, RUDN University, Pirogov Medical University
Russian Federation, 115478, Moscow; 117998, Moscow; 117997, MoscowReferences
- Shannon-Lowe C., Rickinson A. The global landscape of EBV-associated tumors. Front. Oncol. 2019; 9: 713. https://doi.org/10.3389/fonc.2019.00713
- Graham B.S., Lynch D.T. Burkitt Lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Tsao S.W., Tsang C.M., Lo K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017; 372(1732): 20160270. https://doi.org/10.1098/rstb.2016.0270
- Bei J.X., Zuo X.Y., Liu W.S., Guo Y.M., Zeng Y.X. Genetic susceptibility to the endemic form of NPC. Chin. Clin. Oncol. 2016; 5(2): 15. https://doi.org/10.21037/cco.2016.03.11
- Su W.H., Hildesheim A., Chang Y.S. Human leukocyte antigens and Epstein–Barr virus-associated nasopharyngeal carcinoma: old associations offer new clues into the role of immunity in infection-associated cancers. Front. Oncol. 2013; 3: 299. https://doi.org/10.3389/fonc.2013.00299
- Rickinson A.B., Young L.S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J. Virol. 1987; 61(5): 1310–7. https://doi.org/10.1128/jvi.61.5.1310-1317.1987
- Romero-Masters J.C., Huebner S.M., Ohashi M., Bristol J.A., Benner B.E., Barlow E.A., et al. B cells infected with Type 2 Epstein–Barr virus (EBV) have increased NFATc1/NFATc2 activity and enhanced lytic gene expression in comparison to Type 1 EBV infection. PLoS Pathog. 2020; 16(2): e1008365. https://doi.org/10.1371/journal.ppat.1008365
- Correa R.M., Fellner M.D., Alonio L.V., Durand K., Teyssié A.R., Picconi M.A. Epstein–Barr virus (EBV) in healthy carriers: Distribution of genotypes and 30 bp deletion in latent membrane protein-1 (LMP-1) oncogene. J. Med. Virol. 2004; 73(4): 583–8. https://doi.org/10.1002/jmv.20129
- Srivastava G., Wong K.Y., Chiang A.K., Lam K.Y., Tao Q. Coinfection of multiple strains of Epstein–Barr virus in immunocompetent normal individuals: reassessment of the viral carrier state. Blood. 2000; 95(7): 2443–5.
- Van Baarle D., Hovenkamp E., Kersten M.J., Klein M.R., Miedema F., van Oers M.H. Direct Epstein–Barr virus (EBV) typing on peripheral blood mononuclear cells: no association between EBV type 2 infection or superinfection and the development of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma. Blood. 1999; 93(11): 3949–55.
- Sixbey J.W., Shirley P., Chesney P.J., Buntin D.M., Resnick L. Detection of a second widespread strain of Epstein–Barr virus. Lancet. 1989; 2(8666): 761–5. https://doi.org/10.1016/s0140-6736(89)90829-5
- Edwards R.H., Seillier-Moiseiwitsch F., Raab-Traub N. Signature amino acid changes in latent membrane protein 1 distinguish Epstein–Barr virus strains. Virology. 1999; 261(1): 79–95. https://doi.org/10.1006/viro.1999.9855
- Cheung S.T., Leung S.F., Lo K.W., Chiu K.W., Tam J.S., Fok T.F., et al. Specific latent membrane protein 1 gene sequences in type 1 and type 2 Epstein-Barr virus from nasopharyngeal carcinoma in Hong Kong. Int. J. Cancer. 1998; 76(3): 399–406. https://doi.org/10.1002/(sici)1097-0215(19980504)76:3<399:aid-ijc18>3.0.co;2-6
- Oshima M., Azuma H., Okuno A. High prevalence of Epstein-Barr virus type A strain with the 30 b.p. deletion of the latent membrane protein-1 gene in a Japanese population. Pediatr. Int. 1999; 41(5): 490–5. https://doi.org/10.1046/j.1442-200x.1999.01122.x
- Khanim F., Yao Q.Y., Niedobitek G., Sihota S., Rickinson A.B., Young L.S. Analysis of Epstein–Barr virus gene polymorphisms in normal donors and in virus-associated tumors from different geographic locations. Blood. 1996; 88(9): 3491–501.
- Smirnova K.V., Senyuta N.B., Botezatu I.V., Dushen’kina T.E., Lubenskaya A.K., Frolovskaya A.A., et al. Epstein–Barr virus in the ethnic Tatars population: the infection and sequence variants of LMP1 oncogene. Uspekhi molekulyarnoy onkologii. 2018; 5(3): 65–74. https://doi.org/10.17650/2313-805X-2018-5-3 https://elibrary.ru/vloxpu (in Russian)
- Hahn P., Novikova E., Scherback L., Janik C., Pavlish O., Arkhipov V., et al. The LMP1 gene isolated from Russian nasopharyngeal carcinoma has no 30-bp deletion. Int. J. Cancer. 2001; 91(6): 815–21. https://doi.org/10.1002/1097-0215(200002)9999:9999<::aid-ijc1122>3.0.co;2-w
- Hassan R., White L.R., Stefanoff C.G., de Oliveira D.E., Felisbino F.E., Klumb C.E., et al. Epstein–Barr virus (EBV) detection and typing by PCR: a contribution to diagnostic screening of EBV-positive Burkitt’s lymphoma. Diagn. Pathol. 2006; 1: 17. https://doi.org/10.1186/1746-1596-1-17
- Salahuddin S., Khan J., Azhar J., B. Whitehurst C., Qadri I., Shackelford J., et al. Prevalence of Epstein–Barr Virus Genotypes in Pakistani Lymphoma Patients. Asian Pac. J. Cancer Prev. 2018; 19(11): 3153–9. https://doi.org/10.31557/APJCP.2018.19.11.3153
- Kaprin A.D., Starinskiy V.V., Shakhzadova A.O., eds. Malignant Neoplasms in Russia in 2020 (Morbidity and Mortality) [Zlokachestvennye novoobrazovaniya v Rossii v 2020 godu (zabolevaemost’ i smertnost’)]. Moscow; 2021. (in Russian)
- Young L.S., Yao Q.Y., Rooney C.M., Sculley T.B., Moss D.J., Rupani H., et al. New type B isolates of Epstein–Barr virus from Burkitt’s lymphoma and from normal individuals in endemic areas. J. Gen. Virol. 1987; 68(Pt. 11): 2853–62. https://doi.org/10.1099/0022-1317-68-11-2853
Supplementary files