Seroprevalence of herpes simplex virus type 1 (Herpesviridae: Simplexvirus: Human alphaherpesvirus 1) in smokers
- Authors: Mays J.B.1, Mariem M.N.1, Alabadi H.I.2
-
Affiliations:
- University of Basrah
- Basrah Health Directorate
- Issue: Vol 69, No 2 (2024)
- Pages: 187-192
- Section: SHORT COMMUNICATION
- URL: https://virusjour.crie.ru/jour/article/view/16603
- DOI: https://doi.org/10.36233/0507-4088-220
- EDN: https://elibrary.ru/hcmnwk
- ID: 16603
Cite item
Full Text
Abstract
Introduction. Herpes simplex virus type 1 (HSV-1) is one of the most common human viral infections and has a double-stranded DNA genome belonging to the Herpesviridae family. Smoking is one of the leading causes of disease and premature death worldwide, responsible for the death of up to six million people annually. The purpose of the current study was to determine the seroprevalence of HSV-1 infection among smokers.
Methods. The search strategy was conducted in the period from December 2022 to January 2023. The study included a random sample of 94 (88 males, and 6 females) healthy participants, aged between ≤ 20 to ≥ 60 years, with 50 participants as the control group. The HSV serological testing consisted of detecting antibodies to HSV-1 IgG with the help of ELISA.
Results. Most participants were university students, consisting of 45.7% males and 5.3% females, followed by employed smokers, consisting of 0.2% males and 1.1% females. The number of females was much lower than that of males reaching 6.4 and 93.6% respectively, due to customs and traditions. The seroprevalence was 24.47, 22.3 and 2.1% in males and females respectively. The seroprevalence rate was 13.8% in hookah and cigarette smokers, 9% in cigarette smokers and 1.1% in hookah smokers exclusively. The highest rate was observed in the age groups of 21-30 and 31–40 years with 12.80% and 7.40% respectively.
Conclusions. The study revealed that the seroprevalence of HSV-1 IgG was 24.47%, and was higher among hookah and cigarette smokers compared to those who exclusively smoked cigarettes or hookah.
Keywords
Full Text
Introduction
Herpes simplex virus type 1 (HSV-1) is a worldwide infection [1]. HSV-1 is one of the most common human viral pathogens and has a double-stranded DNA genome belonging to the Herpesviridae family [2–4]. The virus, which can cause lifelong infection, is mainly transmitted orally, through direct contact with body fluids and through contact with an infected individual [5, 6]. There are three subfamilies of herpesviruses: alpha, beta, and gamma. HSV-1 is classified as part of the Alphaherpesvirinae subfamily [7–9].
The virus can infect people of all ages and cause persistent and recurrent infections [10, 11]. The first instance of HSV-1 infection appears as febrile vesicles on facial skin, which are called herpes labials or fever blisters, and also commonly known as cold sores on the lips [4, 12]. HSV-1 infection acquisition typically takes place in childhood, well before sexual debut, and is made more likely by close contact with parents, other family members and classmates [6, 13]. Many diseases are caused by HSV-1 infection, however, oral herpes is the most prevalent clinical presentation [5, 6, 13]. A number of studies in many countries have indicated that HSV-1 is mostly contracted through the genitals, rather than through the mouth and it has also been observed that a large number of young people are exposed to the virus for the first time through oral sex [6, 14–22].
Although there is possibly a link between smoking cigarettes and herpes virus infection, it is especially concerning in people who smoke cigarettes more frequently. Smoking is one of the leading causes of disease and premature death worldwide, responsible for the death of up to six million people annually. The World Health Organization (WHO) released statistics showing that by 2030, there will be as many as eight million deaths [23]. According to previous studies, there are 700 million smokers in developing nations overall. In total, 6 trillion cigarettes are smoked annually around the world, with men accounting for 47% of smokers and women for 12% [24]. The purpose of the current study was to determine the seroprevalence of HSV-1 infection among smokers.
Methods
Study design and serological testing
The study was designed to assess the seroprevalence of HSV-1 IgG in smokers. The survey population is a random sample of individuals who participated. The search strategy was conducted in the period from December 2022 to January 2023. The study included a random sample of 94 (88 males, 6 females) healthy participants, aged between ≤ 20 to ≥ 60 years, with 50 participants as the control group. The HSV serological testing was conducted on five milliliters of venous blood specimens that were drawn from each individual participating for the detection of IgG antibodies to HSV-1. Laboratory procedures involved centrifuging serum samples at 5000 RPM for five minutes at 4 °C and the serum was stored in Eppendorf tubes and maintained deeply frozen at −20 °C until testing [25]. The HerpeSelect 1 enzyme-linked immunosorbent assay (ELISA) commercial kit (SunLong Biotech Co., LTD.) was used to conduct HSV-1 serology testing according to the manufacturer’s instructions.
Ethical approval and informed consent
Before collecting samples, Informed consent was obtained from all individuals and a survey was conducted to collect information on the type, duration and chronic diseases of smoking, as well as occupation. The research protocol was approved by the Ethics Committee of the Microbiology Department, College of Medicine, University of Basrah (Protocol No. 030401-042-2023 dated 25/6/2022).
Statistical analyses
Microsoft Excel 2013 was used to store the information collected through the survey results. A normal distribution test was performed on quantitative variables, which were then given as means of standard deviation (SD). Qualitative variables were displayed as percentages. Data were analyzed through Version 26 of the Statistical Package for the Social Sciences (SPSS Inc.) software. The Chi-square test (χ2-test) was applied to determine the statistical significance of the data difference. Statistical significance is set at p < 0.05.
Results
Table 1. Demographic characteristics of the individuals who participated in this study
Age Group (years) Возрастная группа (годы) | Gender Пол | Total Всего (%) | |
male мужской (%) | female женский (%) | ||
≤ 20 | 8.5 | 1.1 | 9.6 |
21–30 | 47.9 | 2.1 | 50.0 |
31–40 | 13.8 | 2.1 | 16.0 |
41–50 | 9.6 | 1.1 | 10.6 |
51–60 | 4.3 | 0.0 | 4.3 |
>60 | 9.6 | 0.0 | 9.6 |
Total Всего | 93.6 | 6.4 | 100.0 |
Occupation Профессия | |||
Employed Работающий | 20.2 | 1.1 | 21.28 |
Student Студент | 45.7 | 5.3 | 51.06 |
Unemployed Безработный | 19.1 | 0.0 | 19.15 |
Retired Пенсионер | 8.5 | 0.0 | 8.51 |
Total Всего | 93.62 | 6.38 | 100.00 |
No. positive of HSV-1% Число положительных по ВПГ-1, % | 22.3 | 2.1 | 24.47 |
Mean ± SD Среднее ± SD | 25 ± 13.57 | ||
p-value значение p | 0.5 | ||
Note. SD ‒ Standard deviation.
Примечание. SD ‒ стандартное отклонение.
Table 1 provides a breakdown of the demographic characteristics of participants in the study and is divided into two sections (gender and age group). The ages of the participants ranged between ≤ 20 and ≥ 60 years and were distributed into six categories. The overall average age of participants was 25 ± 13.57 years while the average age of healthy participants was 25 ± 13.15 years. There were 94 participants, with 93.6% (88) being males and 6.4% (6) being females, as well as healthy participants with 94% (47) and 6% (3) males and females respectively. The age group of 21 to 30 years of age had the highest participation rate (50%) of smokers in this study. Most participants were university students 45.7% of which were males and 5.3% – females, followed by employed smokers, consisting of 0.2% males and 1.1% females. The number of females was much lower than that of males reaching 6.4% and 93.6% respectively, due to customs and traditions (Table 1).
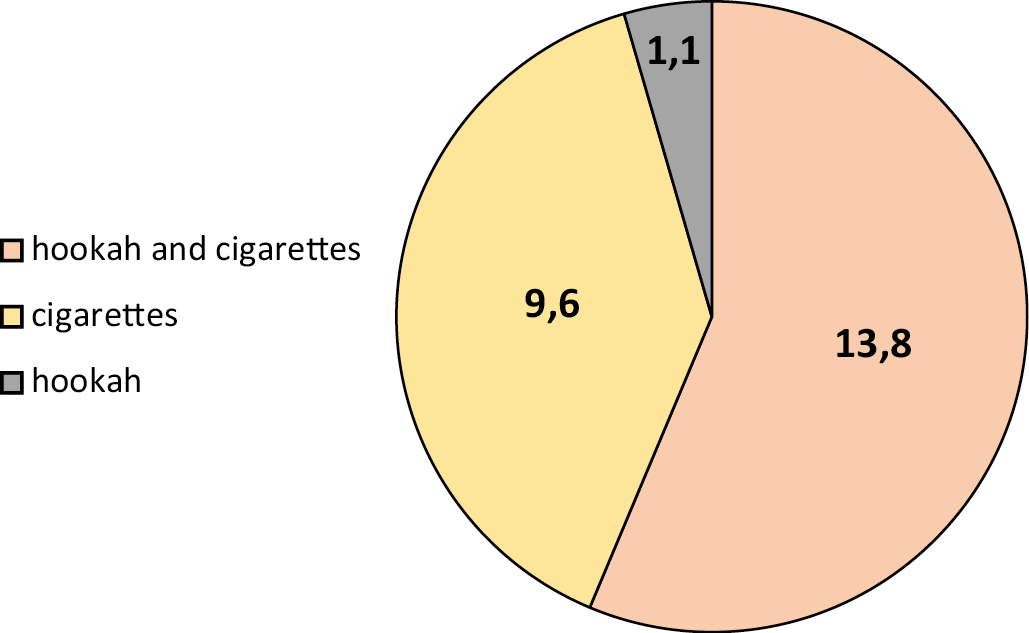
Fig. 1. Distribution of study participants according to the type of smoking.
Рис. 1. Распределение участников исследования по типу курения.
Among the 94 selected samples, the seroprevalence for HSV-1 was 24.47, 22.3 and 2.1% in males and females respectively. The seroprevalence rate was 13.8% in hookah and cigarette smokers, 9% in cigarette smokers and 1.1% in hookah smokers exclusively (Figure 1), and was a highest rate in the age groups 21–30 and 31–40 years with 12.80% and 7.40% respectively (Figure 2).
Fig. 2. Distribution of study participants according to the age groups.
Рис. 2. Распределение участников исследования по возрастным группам
According to gender, a sizable fraction of hookah and cigarette smokers took part in this survey, where a rate of males 32.98% and females 1.06%. The percentage of cigarette smokers was 40.43% among males and 1.06% among females. The percentage for hookah and vape users among males was 3.19% and 1.06% among females. Hookah users among males and females were 14.89% and 3.19% respectively. and for Vape, hookah, and cigarette users was 1.06 % for males and females (Figure 3).
Fig. 3. Distribution of study participants according to gender.
Рис. 3. Распределение участников исследования по полу.
Discussion
Herpes simplex virus type 1 infection is lifelong and it is typically contracted orally and during childhood [13]. The infection can cause serious illness, including severe problems with the nervous system, the eye and the mucous membranes, even though it is almost always mild or asymptomatic [5, 13]. Given that cigarette smoking is one of the most significant possible causes of disrupting the immune system’s function by altering various immunological pathways, there is a close correlation between herpesvirus infection and host immune system response of smokers [26]. However, the effect of smoking on the rate of infection with the herpes virus has not been investigated in many studies.
The findings of the current study differed from those of earlier studies on various demographic groups carried out in various countries. The study showed that there is a paucity of research on the seroprevalence of HSV-1 infection in Iraq. The study found that the seroprevalence was 24.47% for HSV-1, 22.3 and 2.1% for males and females respectively. The HSV-1 seroprevalence rate was 13.8% in hookah and cigarette smokers, 9% in cigarette smokers and 1.1% in hookah smokers exclusively. The highest seroprevalence rate of HSV-1 IgG was in the age groups 21–30 and 31–40 years amounting to 12.80 and 7.40% respectively. Urban studies of females in Uganda and Nigeria revealed high prevalence of HSV-1 infection at 98% and 96.6% respectively [27, 28]. According to the findings of previous studies, the prevalence rates of HSV-1 in the US, Canada and Northern Europe were 58, 51 and 57.7% respectively [29, 30]. When a population-based survey (NHANES III) was carried out from 1988 to 1994, it revealed the prevalence rate of HSV-2 infection in France and the United States amounting to 17.2 and 21.9% respectively [31]. HSV-1 was 68% prevalent globally in 2012, according to the WHO, with Africa having the highest frequency at 87% of the virus [3]. Debrah et al.’s study of women revealed that 99.2% of them had HSV-1 infection, which is a very high prevalence [32]. Adults in Europe have a seroprevalence of 20 to 100% for HSV-1. The average seroprevalence of HSV-1 in Western Europe, Southern Europe, Eastern Europe and Turkey, according to a study conducted in European subregions/countries, was 66.1, 77.2, 78.7 and 87.9%, respectively [30]. According to a previous study, approximately 80% of Australians have the HSV-1 virus, compared to lower prevalence rates in Western nations like Europe (74%), as well as higher prevalence rates in Asia [33], Latin America and the Caribbean [29], Africa [34], the Middle East and North Africa [35], as well as Asia [33], Latin America and the Caribbean [29].
Due to the fact that herpes infection can spread silently across the population and can have serious consequences as a result of the infection development and the emergence of its complications, it is important to increase awareness of these illnesses among the general public. In the current study, University students made up the majority of participants, followed by employees, and more men than women participated because the parents of female university students were unaware that they smoked, which was another factor in why they chose not to participate. The study revealed that the seroprevalence of HSV-1 IgG was higher among hookah and cigarette smokers compared to those who smoked cigarettes or hookah exclusively. Numerous factors, including the geographic location, the number of samples and the inspection process, may be responsible for the virus spreading at this rate in comparison to other nations.
Conclusions
In the current study, the seroprevalence for HSV-1 IgG was 24.47%. University students made up the majority of participants, followed by employed individuals. More men than women participated because the parents of female university students were unaware that they smoked, which was another reason why they chose not to participate. The study revealed that the seroprevalence of HSV-1 IgG was higher among hookah and cigarette smokers compared to those who smoke cigarettes or hookah exclusively.
Acknowledgments
The researchers would like to thank everyone who contributed to the study as well as those who offered assistance.
About the authors
Jalil B. Mays
University of Basrah
Author for correspondence.
Email: medicalresearch20@yahoo.com
ORCID iD: 0000-0002-4799-0879
Assistant Professor, Principle investigator, Department of Microbiology, College of Medicine
Iraq, BasrahMohammed Ali N. Mariem
University of Basrah
Email: mariem.nbeel@uobasrah.edu.iqea
Assistant lecturer, Investigator, Department of Microbiology, College of Medicine
Iraq, Basrah
Hadi I. Alabadi
Basrah Health Directorate
Email: drhadilazim345@gmail.com
ORCID iD: 0000-0003-7884-1224
Physician, Researcher
Iraq, BasrahReferences
- Kasubi M.J., Nilsen A., Marsden H.S., Bergström T., Langeland N., Haarr L. Prevalence of antibodies against herpes simplex virus types 1 and 2 in children and young people in an urban region in Tanzania. J. Clin. Microbiol. 2006; 44(8): 2801–7. https://doi.org/10.1128/jcm.00180-06
- Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., et al. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007.
- Looker K.J., Magaret A.S., May M.T., Turner K.M., Vickerman P., Gottlieb S.L., et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015; 10(10): e0140765. https://doi.org/10.1371/journal.pone.0140765
- Crimi S., Fiorillo L., Bianchi A., D’Amico C., Amoroso G., Gorassini F., et al. Herpes virus, oral clinical signs and QoL: systematic review of recent data. Viruses. 2019; 11(5): 463. https://doi.org/10.3390/v11050463
- Brady R.C., Bernstein D.I. Treatment of herpes simplex virus infections. Antiviral Res. 2004; 61(2): 73–81. https://doi.org/10.1016/j.antiviral.2003.09.006
- Bernstein D.I., Bellamy A.R., Hook E.W. 3rd, Levin M.J., Wald A., Ewell M.G., et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin. Infect. Dis. 2013; 56(3): 344–51. https://doi.org/10.1093/cid/cis891
- Albà M.M., Das R., Orengo C.A., Kellam P. Genomewide function conservation and phylogeny in the Herpesviridae. Genome Res. 2001; 11(1): 43–54. https://doi.org/10.1101/gr.149801
- Davison A.J. Herpesvirus systematics. Vet. Microbiol. 2010; 143(1): 52–69. https://doi.org/10.1016/j.vetmic.2010.02.014
- Zmasek C.M., Knipe D.M., Pellett P.E., Scheuermann R.H. Classification of human Herpesviridae proteins using Domain-architecture Aware Inference of Orthologs (DAIO). Virology. 2019; 529: 29–42. https://doi.org/10.1016/j.virol.2019.01.005
- Looker K.J., Garnett G.P. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Transm. Infect. 2005; 81(2): 103–7. https://doi.org/10.1136/sti.2004.012039
- Mark H.D., Nanda J.P., Roberts J., Rompalo A., Melendez J.H., Zenilman J. Performance of focus ELISA tests for HSV-1 and HSV-2 antibodies among university students with no history of genital herpes. Sex. Transm. Dis. 2007; 34(9): 681–5. https://doi.org/10.1097/01.olq.0000258307.18831.f0
- Leung A.K.C., Barankin B. Herpes labialis: an update. Recent Pat. Inflamm. Allergy Drug Discov. 2017; 11(2): 107–13. https://doi.org/10.2174/1872213x11666171003151717
- Fatahzadeh M., Schwartz R.A. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007; 57(5): 737-63; quiz 764-6. https://doi.org/10.1016/j.jaad.2007.06.027
- Vyse A.J., Gay N.J., Slomka M.J., Gopal R., Gibbs T., Morgan-Capner P., et al. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex. Transm. Infect. 2000; 76(3): 183–7. https://doi.org/10.1136/sti.76.3.183
- Wutzler P., Doerr H.W., Färber I., Eichhorn U., Helbig B., Sauerbrei A., et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations-relevance for the incidence of genital herpes. J. Med. Virol. 2000; 61(2): 201–7. https://doi.org/10.1002/(sici)1096-9071(200006)61:2%3C201::aid-jmv5%3E3.0.co;2-p
- Aarnisalo J., Ilonen J., Vainionpää R., Volanen I., Kaitosaari T., Simell O. Development of antibodies against cytomegalovirus, varicella-zoster virus and herpes simplex virus in Finland during the first eight years of life: a prospective study. Scand. J. Infect. Dis. 2003; 35(10): 750–3. https://doi.org/10.1080/00365540310015881
- Roberts C.M., Pfister J.R., Spear S.J. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex. Transm. Dis. 2003; 30(10): 797–800. https://doi.org/10.1097/01.olq.0000092387.58746.c7
- Pebody R.G., Andrews N., Brown D., Gopal R., De Melker H., François G., et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex. Transm. Infect. 2004; 80(3): 185–91. https://doi.org/10.1136/sti.2003.005850
- Xu F., Lee F.K., Morrow R.A., Sternberg M.R., Luther K.E., Dubin G., et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J. Pediatr. 2007; 151(4): 374–7. https://doi.org/10.1016/j.jpeds.2007.04.065
- Kramer M.A., Uitenbroek D.G., Ujcic-Voortman J.K., Pfrommer C., Spaargaren J., Coutinho R.A., et al. Ethnic differences in HSV1 and HSV2 seroprevalence in Amsterdam, the Netherlands. Euro Surveill. 2008; 13(24): 18904.
- Sauerbrei A., Schmitt S., Scheper T., Brandstädt A., Saschenbrecker S., Motz M., et al., Seroprevalence of herpes simplex virus type 1 and type 2 in Thuringia, Germany, 1999 to 2006. Euro Surveill. 2011; 16(44): 20005.
- Ayoub H.H., Chemaitelly H., Abu-Raddad L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med. 2019; 17(1): 57. https://doi.org/10.1186/s12916-019-1285-x
- WHO. WHO Report on the Global Tobacco Epidemic, 2011: warning about the dangers of tobacco. Geneva; 2011.
- WHO. WHO Report on the Global Tobacco Epidemic, 2008: the MPOWER package. Geneva; 2008.
- Naame Z.K., Thuwaini M.M., Mahdi D.S. Seroprevalence of (Toxoplasma gondii, CMV, Rubella and HSV1&2) in aborted women in Basra, Southern of Iraq. Ann. Trop. Med. Public Health. 2021; 24(5): 295–301.
- Ohana B., Lipson M., Vered N., Srugo I., Ahdut M., Morag A. Novel approach for specific detection of herpes simplex virus type 1 and 2 antibodies and immunoglobulin G and M antibodies. Clin. Diagn. Lab. Immunol. 2000; 7(6): 904–8. https://doi.org/10.1128/cdli.7.6.904-908.2000
- Nakku-Joloba E., Kambugu F., Wasubire J., Kimeze J., Salata R., Albert J.M., et al. Sero-prevalence of herpes simplex type 2 virus (HSV-2) and HIV infection in Kampala, Uganda. Afr. Health Sci. 2014; 14(4): 782–9. https://doi.org/10.4314/ahs.v14i4.2
- Kalu E. Seroprevalence of herpes simplex virus infections among pregnant women attending antenatal clinic in Benin, Nigeria. Int. J. Trop. Dis. Health. 2014; 4(1): 70–81. https://doi.org/10.9734/IJTDH/2014/6048
- Sukik L., Alyafei M., Harfouche M., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in Latin America and the Caribbean: Systematic review and meta-analytics. PLoS One. 2019; 14(4): e0215487. https://doi.org/10.1371/journal.pone.0215487
- Yousuf W., Ibrahim H., Harfouche M., Abu Hijleh F., Abu-Raddad L. Herpes simplex virus type 1 in Europe: systematic review, meta-analyses and meta-regressions. BMJ Glob. Health. 2020; 5(7): e002388. https://doi.org/10.1136/bmjgh-2020-002388
- Fleming D.T., McQuillan G.M., Johnson R.E., Nahmias A.J., Aral S.O., Lee F.K., et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 1997; 337(16): 1105–11. https://doi.org/10.1056/nejm199710163371601
- Debrah O., Agyemang-Yeboah F., Asmah R.H., Timmy-Donkoh E., Seini M.M., Fondjo L.A., et al. SERO-prevalence of herpes simplex virus type 1 and type 2 among women attending routine Cervicare clinics in Ghana. BMC Infect. Dis. 2018; 18(1): 378. https://doi.org/10.1186/s12879-018-3288-1
- Khadr L., Harfouche M., Omori R., Schwarzer G., Chemaitelly H., Abu-Raddad L.J. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin. Infect. Dis. 2019; 68(5): 757–72. https://doi.org/10.1093/cid/ciy562
- Harfouche M., Chemaitelly H., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in Africa: Systematic review, meta-analyses, and meta-regressions. J. Infect. 2019; 79(4): 289–99. https://doi.org/10.1016/j.jinf.2019.07.012
- Chaabane S., Harfouche M., Chemaitelly H., Schwarzer G., Abu-Raddad L.J. Herpes simplex virus type 1 epidemiology in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci. Rep. 2019; 9(1): 1136. https://doi.org/10.1038/s41598-018-37833-8
Supplementary files