Vpr, accessory protein of human immunodeficiency virus type 1 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1): features of genetic variants of the virus circulating in the Moscow region in 2019–2020
- Authors: Kuznetsova A.I.1, Antonova A.A.1, Makeeva E.A.2, Kim K.V.1, Munchak I.M.1, Mezhenskaya E.N.1, Orlova-Morozova E.A.3, Pronin A.Y.3, Prilipov A.G.1, Galzitskaya O.V.1,4
-
Affiliations:
- National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
- Moscow Polytechnic University
- Center for the Prevention and Control of AIDS and Infectious Diseases
- Institute of Theoretical and Experimental Biophysics RAS
- Issue: Vol 70, No 4 (2025)
- Pages: 324-339
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16720
- DOI: https://doi.org/10.36233/0507-4088-296
- EDN: https://elibrary.ru/mfgcsm
- ID: 16720
Cite item
Abstract
Introduction. Vpr is a multifunctional auxiliary HIV-1 protein. Oligomerisation is a prerequisite for the entry of Vpr into the virion and its subsequent participation in the early stages of HIV-infection. To date, natural amino acid substitutions in Vpr associated with disease progression were identified; the possibility of creating therapeutics based on Vpr is being considered.
The aim of the study is to investigate Vpr features in the most common genetic variants of HIV-1 circulating in the Moscow region in 2019–2020.
Materials and methods. HIV-1 samples obtained from 231 patients of the AIDS Prevention and Control Center in the period 2019–2020 were studied according to the scheme: proviral DNA extraction, amplification of the vpr gene, sequencing, and data analysis. Consensus Vpr sequences of the most common genetic variants in Russia and their spatial structures, variability of Vpr variants of HIV-1 sub-subtype A6 in patients with different stages of the disease were studied.
Results. Features of Vpr protein in different genetic variants of HIV-1 could influence the formation of their oligomeric forms. No sites with statistically significant differences in the frequency of amino acid substitutions were identified in patients with different stages of disease.
Conclusion. Vpr protein of HIV-1 genetic variants circulating in Russia may have differences in functional properties. Vpr-A6 variants had low variability in patients with different stages of the disease, and therefore Vpr-A6 can be considered as a target for the development of therapeutic agents.
Keywords
Full Text
Introduction
The Vpr auxiliary protein of human immunodeficiency virus type 1 (Retroviridae: Orthoretrovirinae: Lentivirus: Human immunodeficiency virus-1; HIV-1) is a highly conserved viral protein consisting of 96 amino acid residues (aa), with a mass of approximately 14 kDa [1]. In its structure, the Vpr protein contains three α-helices, which are formed by segments of the protein chain from residue 17 to 33, from residue 38–50, and from residue 55 to 77 (Fig. 1). The α-helices are left-handed and oriented with respect to each other in such a way as to ensure interactions between the following amino acid residues: L20, L23, L26, A30, V31 of the first α-helix, W38, L39, L42, I46 of the second α-helix, and V57, L60, I61, L64, L68, F72 of the third α-helix. The aforementioned orientation is further stabilized by interactions between T19, L20, W54 on one side and H33, F34, H71, F72 on the other. The structure of the Vpr protein is characterized by flexible N- and C-terminal regions: from residues 1 to 13 and from residues 78 to 96, respectively [2].
Fig. 1. Schematic representation of Vpr primary structure.
M – methionine; E – glutamic acid; D – aspartic acid; T – threonine; L – leucine; A – alanine; V – valine; H – histidine; F – phenylalanine; I – isoleucine; Y – tyrosine; W – tryptophan; R – arginine; S – serine; Vpr T-Helper/CD4+ Epitope region (major) – Vpr region in which predominantly the epitopes have been mapped that are recognized by the immune system for subsequent development of CD4+ T cell response; Vpr CTL/CD8+ Epitope region (major) – Vpr region in which predominantly the epitopes have been mapped that are recognized by the immune system for subsequent development of CD8+ cytotoxic T cell response (https://www.hiv.lanl.gov/content/immunology/maps/ctl/Vpr.html).
Рис. 1. Схематическое изображение первичной структуры белка Vpr.
M – метионин; E – глутаминовая кислота; D – аспарагиновая кислота; T – треонин; L – лейцин; A – аланин; V – валин; H – гистидин; F – фенилаланин; I – изолейцин; Y – тирозин; W – триптофан; R – аргинин; S – серин; Vpr T-Helper/CD4+ Epitope region (major) – область белка Vpr, в которой преимущественно были картированы эпитопы, распознающиеся иммунной системой для последующего развития CD4+-T-клеточного ответа (https://www.hiv.lanl.gov/content/immunology/maps/helper/Vpr.html); Vpr CTL/CD8+ Epitope region (major) – область белка Vpr, в которой преимущественно были картированы эпитопы, распознающиеся иммунной системой для последующего развития CD8+-цитотоксического Т-клеточного ответа (https://www.hiv.lanl.gov/content/immunology/maps/ctl/Vpr.html).
The Vpr protein gene is expressed at late stages of the HIV-1 life cycle and binds to the viral Pr55Gag precursor protein, which plays an important role in the assembly and production of viral particles. Oligomerization of Vpr is crucial for the recognition of Pr55Gag, after which Pr55Gag-Vpr complexes accumulate in the plasma membrane for subsequent efficient incorporation into virions [3–5]. Thus, during viral infection of the host cell, the Vpr protein penetrates it as part of the virion, allowing it to actively participate in the early stages of viral replication. The Vpr protein has multiple functions (Fig. 2):
- increases the efficiency and accuracy of reverse transcription [6, 7];
- is part of the pre-integration complex (viral DNA, integrase, Vpr protein, etc.), which facilitates the delivery of viral DNA from the cytoplasm to the nucleus for subsequent integration into the host cell genome [6, 7];
- enhances the transcription of proviral DNA [6–8];
- induces ubiquitin/proteasome-dependent degradation of certain cellular proteins, halting the cell cycle in the G2 phase, which contributes to creating a cellular environment optimal for the expression of HIV-1 genes [7, 8];
- induces a response to DNA damage, which is also thought to potentially lead to cell cycle arrest and increased production of inflammatory cytokines [6, 7];
- violates mitochondrial function, which triggers a series of processes that can also lead to apoptosis [9];
Fig. 2. Activities of Vpr protein.
RT – reverse transcription; Vpr – Vpr protein; Env – Env protein; PIC – pre-integration complex.
Рис. 2. Активности белка Vpr.
ОТ – обратная транскрипция; Vpr – белок Vpr; Env – белок Env; PIC – прединтеграционный комплекс.
In macrophages, Vpr counteracts a specific cellular protein, LAMPT5, which transports the viral protein Env to the lysosome [6, 10].
Vpr is released by producing cells and penetrates the surrounding B lymphocytes. In B-lymphocytes, the Vpr protein affects antibody diversification and has the ability to reduce immunoglobulin class switching [11]. A detail of particular interest is the role of Vpr in enhancing viral infection in non-dividing myeloid cells, macrophages, and dendritic cells, which allow for the formation and maintenance of a viral reservoir, effectively transmitting HIV-1 to CD4+ T cells during antigen presentation [6, 12].
The Vpr protein is considered one of the factors contributing to the development of HIV-associated neurocognitive disorders (HAND) in patients: Vpr can penetrate nerve tissue cells, acts as a neurotoxin that induces apoptosis, activates viral replication in latently infected cells; in neurons, it disrupts the regulation of levels of certain microRNAs and their corresponding genes, which can also cause neuronal dysfunction. Moreover, the vpr gene continues to be expressed even with successful antiretroviral therapy (ART) [13–15].
For many years, the issue of the correlation between amino acid substitutions in the Vpr protein and changes in its functional properties has been studied [16, 17]. Natural amino acid substitutions in the Vpr protein of HIV-1 associated with the degree of HAND development in people living with HIV (PLWH) who are on antiretroviral therapy have been identified [18]. Comparison of the genetic diversity of the Vpr protein variants of HIV-1 in patients with rapid disease progression and in patients with long-term absence of disease progression in the absence of ART showed that amino acid substitutions in the Vpr protein may contribute to changes in viral replication kinetics and lead to the observed differences in disease progression [19]. When comparing the C-terminal region of the Vpr protein in HIV-1 subtype B and C variants, subtype-specific amino acid substitutions were identified that may affect the functional properties of the protein [20].
Almost immediately after the discovery of the Vpr protein, the possibilities of creating agents that inhibit HIV-1 replication by counteracting this protein began to be considered: there are a large number of known attempts to create antiretroviral agents based on both natural and synthetic components [17, 21]. Moreover, the Vpr protein contains epitopes recognized by T cells (https://www.hiv.lanl.gov/content/immunology/maps/helper/Vpr.html, ,https://www.hiv.lanl.gov/content/immunology/maps/ctl/Vpr.html), and is considered as an antigen candidate for the development of an anti-HIV vaccine [22].
The most widely spread genetic variant of HIV-1 in Russia for many years remains sub-subtype A6, while in the countries of Europe, Asia, and America, other variants of the virus circulate [23, 24]. Over time, the coexistence and interaction of HIV-1 sub-subtype A6 with less common genetic variants of the virus in Russia (subtype B, circulating recombinant form CRF02_AG, etc.) have led to the formation and spread of other recombinants. Recent studies demonstrate a gradual increase over time in the proportion of recombinant forms in the genetic structure of HIV-1 circulating in the Russian Federation, particularly due to CRF63_02A6 [25]. Furthermore, during the period of 2022–2023, two new forms were identified in Russia: CRF133_A6B and CRF157_A6C [26, 27]. Thus, despite the gradual change in the composition of circulating HIV-1 variants in Russia, the molecular epidemiological profile of HIV infection still retains its uniqueness.
The aim of this study is to investigate the characteristics of the Vpr protein in genetic variants of HIV-1 circulating in the Moscow region in 2019–2020.
Materials and methods
During the study, clinical samples of whole blood from ART-naive (previously untreated) patients with HIV infection from the State Budgetary Healthcare Institution of the Moscow Region «Center for the Prevention and Control of AIDS» (231 samples) were analyzed. During the period from 2019 to 2020, a single blood sample was taken from each patient as part of the CARE project (https://www.careresearch.eu/, accessed on 01.11.2024). All clinical material was collected and used in this study with the informed consent of the patients and based on the approval of the Biomedical Ethics Committee of the N.F. Gamaleya National Research Center for Epidemiology and Microbiology of the Ministry of Health of Russia (protocol No. 16 dated 02.08.2019). At the same time, the following information about the patients was recorded and subsequently analyzed: gender, age, risk factor for infection, date of clinical sample collection, disease stage, viral load (VL) values, and the patient’s immune status (number of CD4+ cells). Table 1 presents the main characteristics of the patients included in the study, depending on the stage of HIV infection, according to the clinical guidelines of the Ministry of Health of Russia1.
Table 1. Main characteristics of people living with HIV (PLWH) included in the study, classified by stage of HIV infection
Таблица 1. Основные характеристики включенных в исследование ЛЖВ, классифицированных по стадии ВИЧ-инфекции
Characteristics Характеристики | Stage 2/stage of initial symptoms 2-я стадия/стадия начальных проявлений | Stage 3/subclinical stage 3-я стадия/ субклиническая стадия | Stage 4/stage of secondary symptoms 4-я стадия/стадия вторичных проявлений |
Total number of patients, abs. Всего пациентов, абс. | 48 | 82 | 101 |
Demographics | Демографические показатели | |||
Males, abs. Мужчины, абс. | 30 | 45 | 71 |
Females, abs. Женщины, абс. | 18 | 37 | 30 |
Age, median age, range Возраст, медиана лет, диапазон | 38 [19; 62] | 38 [21; 70] | 39 [24; 64] |
Infection route, abs. | Путь инфицирования, абс. | |||
Hetero Гетеро | 24 | 59 | 62 |
IDU ПИН | 6 | 11 | 36 |
MSM МСМ | 17 | 9 | 3 |
Unknown Неизвестно | 1 | 3 | 0 |
Laboratory parameters | Лабораторные показатели | |||
Clones/μL CD4, кл/мкл | 599.50 (108–2022) | 474.10 (110–1658) | 229.72 (8–1062) |
Viral load log10 RNA, copies/mL Вирусная нагрузка lg РНК, копий/мл | 5.0 (3.4–7.0) | 4.6 (3.3–6.2) | 5.1 (3.1–6.4) |
Note. IDU – Injecting drug users; MSM – Men having sex with other men.
Примечание. ПИН – потребители инъекционных наркотиков; МСМ – мужчины, практикующие секс с мужчинами.
The extraction of proviral DNA from genomic DNA was performed using the precipitation method [28]. The products of the genome region encoding the vpr gene were obtained using a nested two-round polymerase chain reaction (PCR): external primers – Vif1f (GCAGGTAAGAGAGCAAGCTGAACA) and Vif1r (GTCTCCGCTTCTTCCTGCCATAGGA), internal primers – Vif2f (GCTaCTCTGGAAAGGTGAAGG) and Vif2r (TACAAGGAGTCTTGGGCTGAC). The obtained PCR products were purified using a commercial PCR fragment purification kit – Clean S-Cap (Evrogen, Russia), and then sequenced by the Sanger dideoxy method using the commercial BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, USA) and the primers Vif2p and Vif2o. The assembly and editing of nucleotide sequences of the vpr gene based on the obtained electropherograms were performed using the SeqMan II 6.1 application (DNASTAR Inc., USA).
Preliminary determination of genetic variants of the obtained nucleotide sequences of the vpr gene of HIV-1 was carried out using three specialized programs: COMET HIV-1 (https://comet.lih.lu/) [29], REGA HIV-1 Subtyping Tool (Version 3.46) (https://www.genomedetective.com/app/typingtool/hiv), and jpHMM [30]. Then, to refine the results of the preliminary subtyping, a phylogenetic analysis using the maximum likelihood method was conducted with the IQ-TREE program [31]. Reference sequences for the analysis were downloaded from the international Los Alamos Laboratory database, USA (https://www.hiv.lanl, accessed on 31.10.2024). Pairwise and multiple alignments of the studied and reference sequences were performed using the ClustalW module integrated into the AliView software package [32]. The nucleotide substitution model was selected using the jModelTest v. 2.1.7 program based on the Akaike information criterion (AIC) [33]. The reliability of the inferred phylogenies was assessed using the bootstrap test and the Shimodaira-Hasegawa approximate likelihood ratio test (SH-aLRT) with 1000 bootstrap iterations. Clusters supported by SH-aLRT > 0.9 were considered reliably established. The visualization and graphical processing of the results of the phylogenetic analysis were carried out in the iTOL program (https://itol.embl.de) [34].
At the next stage of the study, consensus sequences of the Vpr protein for the most common genetic variants of HIV-1 were formed and analyzed based on the study results. For this purpose, the obtained nucleotide sequences of the vpr gene were grouped according to their genetic variants. Then the nucleotide sequences of the HIV-1 vpr gene were translated into amino acids using an online translation tool available at the website (https://www.bioinformatics.org/sms2/translate.html). Also, for each analyzed HIV-1 genetic variant, based on the obtained amino acid sequences, common consensus amino acid sequences were formed using the Simple Consensus Maker tool (https://www.hiv.lanl.gov/content/sequence/CONSENSUS/SimpCon.html) and compared with each other and with respect to Vpr_model (the Vpr protein sequence of subtype B, analyzed during the determination of its spatial structure [2]) using the MEGA v. 10.2.2 program. When forming the consensus sequence, insertions were not taken into account; the frequency at which an amino acid (as well as a stop codon or deletion) was considered in the consensus at each position had to be greater than 50%. Using the IsUnstruct program, the location of unstructured regions in the consensus sequences and in Vpr_model [35] was predicted. Using the AlphaFold 2 program (AlphaFold Protein Structure Database), the spatial structure of the consensus sequences and Vpr_model [36] was predicted.
In the Chimera program, the predicted hexameric structures of the analyzed sequences were overlaid with each other and with Vpr_model to determine the most similar structures (https://www.rbvi.ucsf.edu/chimera/).
At the final stage of the study, the variability of the Vpr-A6 protein (the Vpr protein of HIV-1 sub-subtype A6 variants) was examined in patients with different stages of the disease. For this purpose, the obtained amino acid sequences of Vpr-A6 were grouped according to the stage of HIV infection of the patient from whom the sample was obtained. The previously obtained consensus sequence Vpr-A6 was used as a reference, and amino acid substitutions in each patient group were determined relative to it using the MEGA v. 10.2.2 program (www.megasoftware.net). Using the Nonparametric Statistics module from the Statistica 8.0 package (StatSoft Inc., USA), sites with statistically significant differences in frequency among patients with different stages of the disease were identified (p < 0.0012 using the χ2 test with Bonferroni correction).
Results
During pairwise and multiple alignments and quality assessment, 5 out of 231 sequences were excluded from further analysis due to low quality or sequence length (the number of degenerate bases or gaps exceeding 1% of the total sequence length). In the further analysis, 226 nucleotide sequences encoding the vpr gene of HIV-1 were included. All the nucleotide sequences of the vpr gene of HIV-1 (226) obtained during this study were deposited in the international genotype database GenBank under the following accession numbers: PV059601–PV059826.
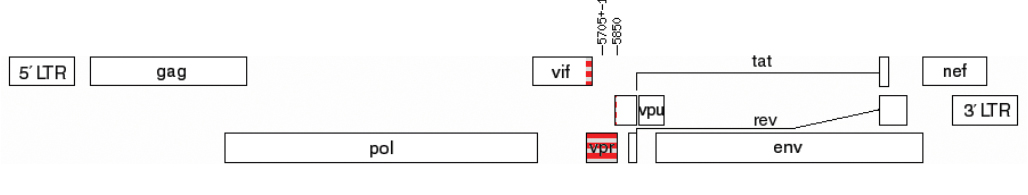
Based on the results of preliminary subtyping, it was determined that two samples (0.88%, 2/226), obtained from patients in the 2nd (1311101072) and 3rd (1311001115) stages of the disease, belonged to unique recombinant forms (URFs) of HIV-1, formed by fragments of HIV-1 genetic variants B and G. The genomic structures of the identified URFs_B/G are presented in Fig. 3.
Fig. 3. Genome map with the studied vpr region in samples 1311001072 (а) и 1311001115 (b).
Рис. 3. Карта генома с исследуемой областью vpr у образцов 1311001072 (а) и 1311001115 (б).
These sequences were excluded from further phylogenetic analysis.
According to the results of the phylogenetic analysis, 4 (1.77%) sequences formed a reliable cluster (SH-aLRT > 0.9) with HIV-1 subtype G sequences, 16 (7.08%) sequences formed a reliable cluster (SH-aLRT > 0.9) with HIV-1 subtype B sequences, and 11 (4.87%) sequences were included in a cluster formed by the nucleotide sequences of circulating recombinant forms CRF02_AG and CRF63_02A6 (Fig. 4).
Fig. 4. Phylogenetic analysis of nucleotide sequences of the HIV-1 vpr gene (n = 254, nucleotide substitution model – TIM1 + I + G4).
Nucleotide sequences classified as potential unique recombinants are marked with a red asterisk.
Рис. 4. Филогенетический анализ нуклеотидных последовательностей гена vpr ВИЧ-1 (n = 254, модель замещения нуклеотидов – TIM1 + I + G4).
Референсные последовательности выделены красным цветом, исследуемые – черным. Красной звездочкой отмечены нуклеотидные последовательности, отнесенные к потенциальным уникальным рекомбинантам.
Since the genetic variants CRF02_AG and CRF63_02A6 in the studied region of the HIV-1 genome (vpr) exhibit maximum similarity, it was decided to identify them based on the combined results of phylogenetic analysis and the COMET HIV-1 program – 10 sequences were classified as CRF63_02A6. Based on the results of the phylogenetic analysis (due to its position on the phylogenetic tree – intermediate from the others) combined with the results of the jpHMM program (for identifying recombinant forms of the virus), it was decided that sequence 1311001105 (marked with a red star in the figure) may be a potential unique recombinant form (URF_CRF02/CRF63). Its genome structure is presented in Fig. 5.
Fig. 5. Genome map for the studied vpr region in sample 1311001105.
The dotted line indicates the region formed by the HIV-1 fragment of recombinant forms CRF02_AG and CRF63_02A6.
Рис. 5. Карта генома с исследуемой областью vpr у образца 1311001105.
Пунктиром отмечена область, образованная фрагментом ВИЧ-1 рекомбинантных форм CRF02_AG и CRF63_02A6.
The sequence 1311000563, based on the combined results of phylogenetic analysis and analysis in the COMET HIV-1 program, was classified as URF_A6/B. The remaining 192 (84.96%) sequences formed a reliable cluster with the nucleotide sequences of HIV-1 sub-subtype A6.
Consensus sequences of the Vpr protein were formed for HIV-1 sub-subtype A6 variants, subtype B, and the recombinant form CRF63_02A6 identified during the study. The consensus amino acid sequence of Vpr-A6 was formed based on 192 studied sequences, subtype B – 16, and CRF63_02A6 – 10, respectively. These genetic variants are also the most common in the territory of the Russian Federation [24].
Insertions (amino acid insertions), deletions (amino acid deletions) and stop codons were identified in the studied amino acid sequences of Vpr-A6. Insertions and deletions were observed in the amino acid sequences obtained from patients with all stages of the disease (Table 2), whereas stop codons were found in 6 patients with stages 3 and 4 of the disease.
Table 2. Identified insertions and deletions in the studied amino acid sequences Vpr-A6
Таблица 2. Выявленные инсерции и делеции в исследуемых аминокислотных последовательностях Vpr-A6
Infection stage Стадия заболевания | Insertions | Инсерции | Deletions | Делеции | ||
sequence name наименование последовательности | position положение | sequence name наименование последовательности | position положение | |
Stage 2 2-я стадия | 1311000412 | ins84V85 | 1311000645 | 85 |
1311000512 | ins84I85 | 1311000738 | 85, 86 | |
1311000948 | 85 | |||
Stage 3 3-я стадия | 1311000660 | ins84I85 | 1311000121 | 85, 86 |
1311000278 | 84 | |||
1311000997 | ins84I85 | 1311000601 | 84 | |
1311000613 | 85, 86 | |||
1311000617 | 85, 86 | |||
1311001126 | ins84I85 | 1311000780 | 85, 86 | |
1311001119 | 85 | |||
1311001125 | 85, 86 | |||
Stage 4 4-я стадия | 1311000884 | ins84P85 | 1311000382 | 87, 88 |
1311000599 | 85, 86 | |||
1311000766 | 85 | |||
1311000767 | 85 | |||
1311000919 | 85 | |||
1311001068 | 85, 89 | |||
1311001088 | 85, 86 | |||
1311001089 | 85 | |||
1311001093 | 85 | |||
All the formed consensus sequences contained 96 amino acid residues (Fig. 6).
Fig. 6. Consensus sequences of Vpr HIV-1 sub-subtype A6, B and CRF63_02A6 genetic variants aligned with the Vpr_model (sequence of the Vpr protein analyzed in determining the spatial structure [2]).
The dots indicate amino acid residues (aa) positions in which the aa in the consensus were the same as in the reference. Non-polar amino acids: G (glycine), A (alanine), V (valine), L (leucine), I (isoleucine), P (proline) – are marked in blue; Polar uncharged amino acids: S (serine), T (threonine), C (cysteine), M (methionine), N (asparagine), Q (glutamine) – green; aromatic amino acids: F (phenylalanine), Tyrosine (Y), W (tryptophan), Histidine (H) – yellow; Polar acidic, negatively charged, amino acids: aspartic acid (D) and glutamic acid (E) – orange; Polar basic, positively charged amino acids: lysine (K), arginine (R) – in red [37, 38].
Рис. 6. Консенсусные последовательности Vpr ВИЧ-1 суб-субтипа А6, субтипа В и рекомбинантной формы CRF63_02A6, выравненные относительно Vpr_model (последовательность белка Vpr, анализируемого при определении пространственной структуры [2]).
Точками обозначены позиции аминокислотных остатков (а.о.), в которых а.о. в консенсусах соответствовали референсу. Аминокислоты классифицированы на основе полярности радикалов. Неполярные аминокислоты: G (глицин), A (аланин), V (валин), L (лейцин), I (изолейцин), P (пролин) отмечены синим цветом; полярные незаряженные аминокислоты: S (серин), T (треонин), C (цистеин), M (метионин), N (аспарагин), Q (глутамин) – зеленым; ароматические аминокислоты: F (фенилаланин), тирозин (Y), W (триптофан), гистидин (H) – желтым; отрицательно заряженные аминокислоты: аспарагиновая кислота (D) и глутаминовая кислота (E) – оранжевым; положительно заряженные аминокислоты: лизин (K), аргинин (R) – красным [37, 38].
The primary structure of the consensus sequences of sub-subtype A6, subtype B, and CRF63_02A6 differed from Vpr_model at positions 17, 11, and 15, respectively (Fig. 6).
Subsequently, for the comparison of spatial structures during the analysis of the consensus sequence of subtype B, we analyzed a sequence variant containing Q at position 77 and I at position 84. For the analysis of the consensus sequence of CRF63_02A6, we analyzed a sequence variant containing R at position 58.
Fig. 7 presents the predicted profiles for unstructured regions for Vpr_model, consensus sequences of sub-subtype A6, subtype B, and CRF63_02A6.
Fig. 7. The comparison of the tertiary structure of the consensus sequences of sub-subtype A6, subtype B and CRF63_02A6 and Vpr_model, predicted by the IsUnstruct program.
a – Vpr_model: unfolded regions from 1 to 15 and from 86 to 96 aa; b – sub-subtype A6 consensus: unfolded regions from 1 to 15 and from 84 to 96 aa; c – subtype B consensus: unfolded regions from 1 to 16 and from 86 to 96 aa; d – CRF63_02A6 consensus: unfolded regions from 1 to 15 and from 86 to 96 aa.
Рис. 7. Сравнение профилей неструктурированных участков для консенсусных последовательностей суб-субтипа А6, субтипа В и CRF63_02A6 и Vpr_model, предсказанные программой IsUnstruct.
a – Vpr_model: развернутые участки с 1–15 и с 86–96 а.о.; б – консенсус суб-субтипа А6: развернутые участки с 1–15 и с 84–96 а.о.; в – консенсус субтипа В: развернутые участки с 1–16 и с 86–96 а.о.; г – консенсус CRF63_02A6: развернутые участки с 1–15 и с 86–96 а.о.
Fig. 8 presents the results of predicting the spatial structure of monomeric, dimeric, tetrameric and hexameric structures of the analyzed Vpr protein sequences.
Fig. 8. Monomeric, dimeric and oligomeric forms of Vpr protein in Vpr_model, sub-subtype A6, subtype B and CRF63_02A6 variants predicted by the AlphaFold 2 program.
a – monomeric forms of Vpr protein; b – dimeric forms of Vpr protein; c – tetrameric forms of Vpr protein; d – hexameric forms of Vpr protein.
Рис. 8. Мономерные, димерные и олигомерные формы белка Vpr вариантов Vpr_model, суб-субтипа А6, субтипа В, рекомбинантной формы CRF63_02A6, предсказанные программой AlphaFold 2.
a – мономерные формы белка Vpr; б – димерные формы белка Vpr; в – тетрамерная форма белка Vpr; г – гексамерная форма белка Vpr.
The results of the spatial alignment (matching) of the predicted hexameric structures are presented in Fig. 9.
Fig. 9. Alignment of Vpr hexameric structures.
Hexamer A6 and Hexamer Vpr_model – hexamer of A6 consensus sequence and hexamer Vpr_model; Hexamer B and Hexamer Vpr_model – hexamer of subtype B consensus sequence and hexamer Vpr_model; Hexamer CRF63_02A6 and Hexamer Vpr_model – hexamer of CRF63_02A6 consensus sequence and hexamer Vpr_model; Hexamer A6 and Hexamer B – hexamer of A6 consensus sequence and hexamer of subtype B consensus sequence; Hexamer A6 and Hexamer CRF63_02A6 – hexamer of A6 consensus sequence and hexamer of CRF63_02A6 consensus sequence; Hexamer B and Hexamer CRF63_02A6 – hexamer of subtype B consensus sequence and hexamer of CRF63_02A6 consensus sequence; The root mean square deviation between Cα atoms for different pairs of hexamers is shown in the figure, which varies from 16.9 Å to 37.8 Å.
Рис. 9. Совмещение гексамерных структур Vpr.
Hexamer A6 and Hexamer Vpr_model – гексамер консенсусной последовательности белка суб-субтипа А6 и гексамер Vpr_model; Hexamer B and Hexamer Vpr_model – гексамер консенсусной последовательности белка субтипа B и гексамер Vpr_model; Hexamer CRF63_02A6 and Hexamer Vpr_model – гексамер консенсусной последовательности рекомбинантной формы CRF63_02A6 и гексамер Vpr_model; Hexamer A6 and Hexamer B – гексамер консенсусной последовательности суб-субтипа А6 и гексамер консенсусной последовательности субтипа B; Hexamer A6 and Hexamer CRF63_02A6 – гексамер консенсусной последовательности суб-субтипа А6 и гексамер консенсусной последовательности CRF63_02A6; Hexamer B and Hexamer CRF63_02A6 – гексамер консенсусной последовательности субтипа B и гексамер консенсусной последовательности CRF63_02A6; Среднеквадратичное отклонение между Cα-атомами для разных пар гексамеров показано на рисунке, которое изменяется от 16,9 Å до 37,8 Å.
When assessing the variability of Vpr-A6 in patients with different stages of HIV infection, 14 substitutions were identified that had statistically significant differences (p<0.05) in their frequency of occurrence (Table 3).
Table 3. Vpr-A6 amino acid substitutions with statistically significant differences in frequency of occurrence in groups of PLWH with different stages of the disease
Таблица 3. Аминокислотные замены Vpr-A6 со статистически значимыми различиями по частоте встречаемости в группах ЛЖВ с разными стадиями заболевания
Position Позиция | Mutation Мутация | Stage 2 Стадия 2 | Stage 3 Стадия 3 | Stage 4 Стадия 4 | p2‒3 | p2‒4 | p3–4 |
13 | E13A | 2 | 0 | 0 | 0.0465 | 0.0190 | – |
15 | Y15H | 6 | 8 | 23 | – | – | 0.0444 |
15 | Y15F | 1 | 5 | 1 | – | – | 0.0353 |
19 | M19V | 3 | 0 | 2 | 0.0143 | – | – |
20 | L20I | 0 | 3 | 0 | – | – | 0.0390 |
45 | H45Q | 6 | 6 | 3 | – | 0.0054 | – |
55 | E55V | 2 | 0 | 0 | 0.0465 | 0.0190 | – |
61 | I61T | 6 | 2 | 8 | 0.0107 | – | – |
72 | F72Y | 2 | 0 | 0 | 0.0465 | 0.0190 | – |
77 | Q77H | 5 | 4 | 4 | – | 0.0451 | – |
85 | Q85H | 1 | 0 | 7 | – | – | 0.0219 |
87 | R87S | 5 | 3 | 3 | – | 0.0194 | – |
93 | S93T | 0 | 3 | 0 | – | – | 0.0390 |
94 | S94N | 1 | 3 | 0 | – | – | 0.0390 |
Note. The p-values are presented for items with p < 0.05; items with p ≥ 0.05 are marked with ‘–’. Differences with p-value with Bonferroni correction (p < 0.0012) were considered as statistically significant.
Примечание. Значения p-value представлены для позиций с p < 0,05; позиции с p ≥ 0,05 отмечены знаком «–». Достоверно значимыми считали различия с
Taking into account the Bonferroni correction (p < 0.0012), no site was found to have statistically significant differences in prevalence among patients with different stages of the disease.
Discussion
Currently, the genetic diversity of HIV-1 continues to grow worldwide, which is one of the obstacles to developing effective prevention and treatment methods for HIV infection [39]. Moreover, research results suggest that different genetic variants of HIV-1 can determine various clinical manifestations and the rate of disease progression, as well as influence treatment efficacy [40]. Regular studies are conducted to investigate the extent of the influence of individual viral proteins on the course of HIV infection [14, 41–43]. Amino acid substitutions in viral proteins that may influence the progression of HIV infection are also studied, with particular attention given to subtype-specific amino acid substitutions [18, 44, 45]. In previously conducted studies on the genetic diversity of the Vpr protein of the most widely spread subtype A6 in Russia, its low level of variability was noted, and in this regard, the Vpr protein was identified as a promising target for the development of therapeutic agents [46]. Also, for the Vpr protein of subtype A6, variants of the virus circulating in different regions of Russia have not previously been noted to have characteristic features [47]. The present study aims to investigate the characteristics of the Vpr protein of the most widely spread genetic variants of HIV-1 in Russia, using the example of virus variants circulating in the Moscow region in 2019–2020, and to compare the genetic variability of the Vpr-A6 protein in patients with different stages of the disease.
According to the results of the study, it was found that the majority (84.96%) of the vpr nucleotide sequences belonged to HIV-1 sub-subtype A6, the second most common was subtype B (7.08%), followed by the recombinant form CRF63_02A6 (4.87%), which is consistent with the results of the study of HIV-1 genetic diversity in the Russian Federation [24]. Two sequences were identified as unique B/G recombinant forms, which also aligns with previously reported data on the identification of unique B/G recombinant forms in Russia [48]. One sequence of the vpr gene was identified as a unique A6/B recombinant form, which is supported by data on the formation of various recombinant forms between HIV-1 subtype A6 and subtype B in Russia [49].
Six out of 192 (3.13%) Vpr-A6 sequences obtained from patients at different stages of HIV infection contained an insertion between the 84th and 85th positions of the amino acid residues. Twenty out of 192 (10.42%) Vpr-A6 sequences obtained from patients at different stages of the disease contained deletions at positions 84–89, with 50% of these sequences containing two deletions simultaneously. The most frequent deletions were found at positions 85 (85%, 17/20) and 86 (40%, 8/20) (Table 2). Earlier, the presence of deletions at positions 85, 86 and 89 in the Vpr protein of sub-subtype A6 variants was noted [46]. Premature stop codons were identified in 6 patients with stage 3 and 4 disease. There are known studies on the patterns and frequency of stop codons in regions of proviral DNA encoding protease, reverse transcriptase, and integrase [50, 51]. However, similar studies on the prevalence of stop codons in proviral DNA encoding the Vpr protein have not yet been conducted. Overall, there is currently a hypothesis that defective proviruses may possess biological activity: the transcripts and corresponding proteins formed on their basis may participate in stimulating the immune response, subsequent chronic activation of the immune system, and pose a serious obstacle to the development of HIV eradication strategies [52].
The consensus sequences of the analyzed HIV-1 variants at several positions, presumably involved in the formation of the protein’s spatial structure, contained substitutions relative to the reference sequence – Vpr_model: substitution D17E – sub-subtype A6, subtype B, CRF63_02A6; T19M – sub-subtype A6, T55E – sub-subtype A6, CRF63_02A6; T55A – subtype B; L60I – sub-subtype A6, subtype B, CRF63_02A6 and R77Q – sub-subtype A6, subtype B, CRF63_02A6. At the same time, the substitutions T55E, T55A and R77Q led to changes in the chemical properties of the amino acid residue at the specified position.
Prediction of the spatial structures of consensus sequences and the reference sequence Vpr_model determined that in the analyzed sequences, the structured regions of the protein predominantly fell within the region from residue 16 to 85, which coincided with the region where epitopes were mapped in the Vpr protein (Fig. 1, 7).
The prediction of oligomeric structures of the Vpr protein consensus sequences and Vpr_model demonstrated differences among tetrameric and hexameric forms. When spatially aligning the hexameric forms, it was determined that the highest root mean square deviation (RMSD) between Cα atoms was 37.8 Å for the pair of hexamers of subtype B consensus sequence and CRF63_02A6 consensus sequence, while the lowest was 16.9 Å for the pair of hexamers of recombinant CRF63_02A6 consensus sequence and Vpr_model hexamer (Fig. 9). RMSD is a quantitative measure of similarity between two protein structures, and the lowest RMSD value between oligomeric forms indicates their structural similarity.
Thus, the existing features of the Vpr protein in various HIV-1 variants may influence the formation of oligomeric forms of the protein. Considering the high significance of the oligomerization process, which affects the incorporation of the Vpr protein into virions and, consequently, determines the potential involvement of the Vpr protein in the early stages of viral replication, it can be asserted that the existing features may influence the functional properties of the Vpr protein [4].
In a previously conducted study on the dynamics of vpr gene variability in patients infected with HIV-1 subtype C, a gradual increase in vpr genetic diversity in the virus population of the patient was observed during the first year of disease progression [53]. However, a comparison of the Vpr protein among virus variants isolated from patients at different stages of the disease in China did not reveal significant differences in amino acids in functionally important regions [54]. In this study, no amino acid substitutions were found in the Vpr-A6 protein that had statistically significant differences in frequency among patients at different stages of the disease, which confirms previous conclusions about the low variability of the Vpr-A6 protein and its potential for use in developing HIV infection therapies [46, 47].
The limitations of the conducted study include a relatively small sample size, including non-A6 variants, as well as the study of virus variants circulating within a single region – the Moscow region.
Conclusion
For the first time, a comparison of the characteristics of Vpr proteins of the most widely distributed HIV-1 genetic variants in the Russian Federation (A6, B, CRF63) has been conducted. It has been established that the present features can influence the formation of oligomeric forms of the protein. Considering the importance of the oligomerization process of the Vpr protein, it can be assumed that the existing differences may lead to varying functional activities of the Vpr protein among HIV-1 variants. At the same time, the comparison of genetic diversity of Vpr-A6 in patients at different stages of HIV infection did not reveal statistically significant amino acid substitutions, which confirms data on the low variability of the Vpr protein within HIV-1 sub-subtype A6 variants and the possibility of its application in the development of HIV infection therapy.
1 Ministry of Health of the Russian Federation. Clinical recommendations. HIV infection in adults; 2024. Available at: https://cr.minzdrav.gov.ru/schema/79_2 (in Russian)
About the authors
Anna I. Kuznetsova
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Author for correspondence.
Email: a-myznikova@list.ru
ORCID iD: 0000-0001-5299-3081
PhD, head of laboratory of T-lymphotropic viruses, PhD, leading researcher, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowAnastasiia A. Antonova
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: aantonova1792@gmail.com
ORCID iD: 0000-0002-9180-9846
PhD, Researcher, Laboratory of T-lymphotropic viruses, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowEkaterina A. Makeeva
Moscow Polytechnic University
Email: makeevakaty13@gmail.com
ORCID iD: 0009-0005-7085-3361
student, Faculty of Chemical Engineering and Biotechnology
Russian Federation, 107023, MoscowKristina V. Kim
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: kimsya99@gmail.com
ORCID iD: 0000-0002-4150-2280
junior researcher, Laboratory of T-lymphotropic viruses, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowIana M. Munchak
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: yana_munchak@mail.ru
ORCID iD: 0000-0002-4792-8928
junior researcher, Laboratory of T-lymphotropic viruses, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowEkaterina N. Mezhenskaya
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: belokopytova.01@mail.ru
ORCID iD: 0000-0002-3110-0843
PhD, Researcher, Laboratory of T-lymphotropic viruses, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowElena A. Orlova-Morozova
Center for the Prevention and Control of AIDS and Infectious Diseases
Email: orlovamorozova@gmail.com
ORCID iD: 0000-0003-2495-6501
PhD, Head of outpatient department
Russian Federation, 140053, Kotelniki, Moscow regionAlexander Yu. Pronin
Center for the Prevention and Control of AIDS and Infectious Diseases
Email: alexanderp909@gmail.com
ORCID iD: 0000-0001-9268-4929
PhD, Chief Physician
Russian Federation, 140053, Kotelniki, Moscow regionAlexey G. Prilipov
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya
Email: a_prilipov@mail.ru
ORCID iD: 0000-0001-8755-1419
Doctor of Biological Sciences, leading researcher, head of the laboratory of molecular genetics, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, MoscowOxana V. Galzitskaya
National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya; Institute of Theoretical and Experimental Biophysics RAS
Email: ogalzit@vega.protres.ru
ORCID iD: 0000-0002-3962-1520
Doctor of Physical and Mathematical Sciences, Head of the Bioinformatics Laboratory, Chief Researcher, Gamaleya National Research Center of Epidemiology and Microbiology, D.I. Ivanovsky Institute of Virology
Russian Federation, 123098, Moscow; 142290, Pushchino, Moscow RegionReferences
- Kogan M., Rappaport J. HIV-1 accessory protein Vpr: Relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology. 2011; 8: 25. https://doi.org/10.1186/1742-4690-8-25
- Morellet N., Bouaziz S., Petitjean P., Roques B.P. NMR structure of the HIV-1 regulatory protein VPR. J. Mol. Biol. 2003; 327(1): 215–27. https://doi.org/10.1016/s0022-2836(03)00060-3
- Sawaya B.E., Khalili K., Gordon J., Taube R., Amini S. Cooperative interaction between HIV-1 regulatory proteins Tat and Vpr modulates transcription of the viral genome. J. Biol. Chem. 2000; 275(45): 35209–14. https://doi.org/10.1074/jbc.M005197200
- Fritz J.V., Dujardin D., Godet J., Didier P., De Mey J., Darlix J.L., et al. HIV-1 Vpr oligomerization but not that of Gag directs the interaction between VPR and GAG. J. Virol. 2010; 84(3): 1585–96. https://doi.org/10.1128/JVI.01691-09
- Venkatachari N.J., Walker L.A., Tastan O., Le T., Dempsey T.M., Li Y., et al. Human immunodeficiency virus type 1 Vpr: oligomerization is an essential feature for its incorporation into virus particles. Virol. J. 2010; 7: 119. https://doi.org/10.1186/1743-422X-7-119
- Nodder S.B., Gummuluru S. Illuminating the role of VPR in HIV infection of myeloid cells. Front. Immunol. 2019; 10: 1606. https://doi.org/10.3389/fimmu.2019.01606
- Vanegas-Torres C.A., Schindler M. HIV-1 Vpr functions in primary CD4+ T Cells. Viruses. 2024; 16(3): 420. https://doi.org/10.3390/ v16030420
- Zhang F., Bieniasz P.D. HIV-1 VPR induces cell cycle arrest and enhances viral gene expression by depleting CCDC137. Elife. 2020; 9: e55806. https://doi.org/10.7554/eLife.55806
- Huang C.Y., Chiang S.F., Lin T.Y., Chiou S.H., Chow K.C. HIV-1 VPR triggers mitochondrial destruction by impairing Mfn2-mediated ER-mitochondria interaction. PLoS One. 2012; 7(3): e33657. https://doi.org/10.1371/journal.pone.0033657
- Zhao L., Wang S., Xu M., He Y., Zhang X., Xiong Y., et al. VPR counteracts the restriction of LAPTM5 to promote HIV-1 infection in macrophages. Nat. Commun. 2021; 12(1): 3691. https://doi.org/10.1038/s41467-021-24087-8
- Eldin P., Péron S., Galashevskaya A., Denis-Lagache N., Cogné M., Slupphaug G., et al. Impact of HIV-1 Vpr manipulation of the DNA repair enzyme UNG2 on B lymphocyte class switch recombination. J. Transl. Med. 2020; 18(1): 310. https://doi.org/10.1186/s12967-020-02478-7
- Casey Klockow L., Sharifi H.J., Wen X., Flagg M., Furuya A.K., Nekorchuk M., et al. The HIV-1 protein Vpr targets the endoribonuclease Dicer for proteasomal degradation to boost macrophage infection. Virology. 2013; 444(1-2): 191–202. https://doi.org/10.1016/j.virol.2013.06.010
- Li G., Makar T., Gerzanich V., Kalakonda S., Ivanova S., Pereira E.F.R., et al. HIV-1 VPR-induced proinflammatory response and apoptosis are mediated through the Sur1-Trpm4 channel in astrocytes. mBio. 2020; 11(6): e02939–20. https://doi.org/10.1128/mbio.02939-20
- Mukerjee R., Chang J.R., Del Valle L., Bagashev A., Gayed M.M., Lyde R.B., et al. Deregulation of microRNAs by HIV-1 VPR protein leads to the development of neurocognitive disorders. J. Biol. Chem. 2011; 286(40): 34976–85. https://doi.org/10.1074/jbc.M111.241547
- James T., Nonnemacher M.R., Wigdahl B., Krebs F.C. Defining the roles for VPR in HIV-1-associated neuropathogenesis. J. Neurovirol. 2016; 22(4): 403–15. https://doi.org/10.1007/s13365-016-0436-5
- Fabryova H., Strebel K. VPR and its cellular interaction partners: R we there yet? Cells. 2019; 8(11): 1310. https://doi.org/10.3390/cells8111310
- González M.E. The HIV-1 VPR protein: A multifaceted target for therapeutic intervention. Int. J. Mol. Sci. 2017; 18(1): 126. https://doi.org/10.3390/ijms18010126
- Dampier W., Antell G.C., Aiamkitsumrit B., Nonnemacher M.R., Jacobson J.M., Pirrone V., et al. Specific amino acids in HIV-1 Vpr are significantly associated with differences in patient neurocognitive status. J. Neurovirol. 2017; 23(1): 113–24. https://doi.org/10.1007/s13365-016-0462-3
- Hadi K., Walker L.A., Guha D., Murali R., Watkins S.C., Tarwater P., et al. Human immunodeficiency virus type 1 VPR polymorphisms associated with progressor and non-progressor individuals alter VPR-associated functions. J. Gen. Virol. 2014; 95(3): 700–11. https://doi.org/10.1099/vir.0.059576-0
- Colle J.H., Rose T., Rouzioux Ch., Garcia A. Two highly variable Vpr84 and Vpr85 residues within the HIV-1-Vpr C-terminal protein transduction domain control transductionnal activity and define a clade specific polymorphism. World Journal of AIDS. 2014; (4): 148–55. https://doi.org/10.4236/wja.2014.4201
- Hagiwara K., Ishii H., Murakami T., Takeshima S.N., Chutiwitoonchai N., Kodama E.N., et al. Synthesis of a VPR-binding derivative for use as a novel HIV-1 inhibitor. PLoS One. 2015; 10(12): e0145573. https://doi.org/10.1371/journal.pone.0145573
- Milani A., Baesi K., Agi E., Marouf G., Ahmadi M., Bolhassani A. HIV-1 accessory proteins: which one is potentially effective in diagnosis and vaccine development? Protein Pept. Lett. 2021; 28(6): 687–98. https://doi.org/10.2174/0929866528999201231213610
- Bbosa N., Kaleebu P., Ssemwanga D. HIV subtype diversity worldwide. Curr. Opin. HIV AIDS. 2019; 14(3): 153–60. https://doi.org/10.1097/COH.0000000000000534
- Antonova A.A., Kuznetsova A.I., Ozhmegova E.N., Lebedev A.V., Kazennova E.V., Kim K.V., et al. Genetic diversity of HIV-1 at the current stage of the epidemic in the Russian Federation: an increase in the prevalence of recombinant forms. VICh-infektsiya i immunosupressii. 2023; 15(3): 61–72. https://doi.org/10.22328/2077-9828-2023-15-3-61-72 https://elibrary.ru/tpwttn (in Russian)
- Antonova A., Kazennova E., Lebedev A., Ozhmegova E., Kuznetsova A., Tumanov A., et al. Recombinant forms of HIV-1 in the last decade of the epidemic in the Russian Federation. Viruses. 2023; 15(12): 2312. https://doi.org/10.3390/v15122312
- Maksimenko L.V., Sivay M.V., Totmenin A.V., Shvalov A.N., Skudarnov S.E., Ostapova T.S., et al. Novel HIV-1 A6/B recombinant forms (CRF133_A6B and URF_A6/B) circulating in Krasnoyarsk region, Russia. J. Infect. 2022; 85(6): 702–69. https://doi.org/10.1016/j.jinf.2022.10.001
- Halikov M.R., Ekushov V.E., Totmenin A.V., Gashnikova N.M., Antonets M.E., Tregubchak T.V., et al. Identification of a novel HIV-1 circulating recombinant form CRF157_A6C in Primorsky Territory, Russia. J. Infect. 2024; 88(2): 180–2. https://doi.org/10.1016/j.jinf.2023.11.005
- Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic. Acids. Res. 1988; 16(3): 1215. https://doi.org/10.1093/nar/16.3.1
- Struck D., Lawyer G., Ternes A.M., Schmit J.C., Bercoff D.P. COMET: adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014; 42(18): e144. https://doi.org/10.1093/nar/gku739
- Schultz A.K., Bulla I., Abdou-Chekaraou M., Gordien E., Morgenstern B., Zoaulim F., et al. jpHMM: recombination analysis in viruses with circular genomes such as the hepatitis B virus. Nucleic Acids Res. 2012; 40(Web Server issue): W193–8. https://doi.org/10.1093/nar/gks414
- Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015; 32(1): 268–74. https://doi.org/10.1093/molbev/msu300
- Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014; 30(22): 3276–8. https://doi.org/10.1093/bioinformatics/btu531
- Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012; 9(8): 772. https://doi.org/10.1038/nmeth.2109
- Letunic I., Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021; 49(W1): W293–6. https://doi.org/10.1093/nar/gkab301
- Lobanov M.Y., Sokolovskiy I.V., Galzitskaya O.V. IsUnstruct: prediction of the residue status to be ordered or disordered in the protein chain by a method based on the Ising model. J. Biomol. Struct. Dyn. 2013; 31(10): 1034–43. https://doi.org/10.1080/07391102.2012.718529
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021; 596(7873): 583–9. https://doi.org/10.1038/s41586-021-03819-2
- Berezov T.T., Korovkin B.F. Biological Chemistry [Biologicheskaya khimiya]. Moscow: Meditsina; 1998. (in Russian)
- Lobanov M.Y., Pereyaslavets L.B., Likhachev I.V., Matkarimov B.T., Galzitskaya O.V. Is there an advantageous arrangement of aromatic residues in proteins? Statistical analysis of aromatic interactions in globular proteins. Comput. Struct. Biotechnol. J. 2021; 19: 5960–8. https://doi.org/10.1016/j.csbj.2021.10.036
- Nair M., Gettins L., Fuller M., Kirtley S., Hemelaar J. Global and regional genetic diversity of HIV-1 in 2010-21: systematic review and analysis of prevalence. Lancet Microbe. 2024; 5(11): 100912. https://doi.org/10.1016/S2666-5247(24)00151-4
- Bouman J.A., Venner C.M., Walker C., Arts E.J., Regoes R.R. Per-pathogen virulence of HIV-1 subtypes A, C and D. Proc. Biol. Sci. 2023; 290(1998): 20222572. https://doi.org/10.1098/rspb.2022.2572
- Sami Saribas A., Cicalese S., Ahooyi T.M., Khalili K., Amini S., Sariyer I.K. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017; 8(1): e2542. https://doi.org/10.1038/cddis.2016.467
- Cafaro A., Schietroma I., Sernicola L., Belli R., Campagna M., Mancini F., et al. Role of HIV-1 tat protein interactions with host receptors in HIV infection and pathogenesis. Int. J. Mol. Sci. 2024; 25(3): 1704. https://doi.org/10.3390/ijms25031704
- Khan N., Geiger J.D. Role of Viral Protein U (VPU) in HIV-1 infection and pathogenesis. Viruses. 2021; 13(8): 1466. https://doi.org/10.3390/v13081466
- Ruiz A.P., Ajasin D.O., Ramasamy S., DesMarais V., Eugenin E.A., Prasad V.R. A naturally occurring polymorphism in the HIV-1 tat basic domain inhibits uptake by bystander cells and leads to reduced neuroinflammation. Sci. Rep. 2019; 9(1): 3308. https://doi.org/10.1038/s41598-019-39531-5
- Lebedev A., Kim K., Ozhmegova E., Antonova A., Kazennova E., Tumanov A., et al. Rev protein diversity in HIV-1 group M clades. Viruses. 2024; 16(5): 759. https://doi.org/10.3390/v16050759
- Lapavok I.A. Analysis of polymorphism of non-structural regions in the genome of the HIV-1 variant dominant in Russia: Diss. Moscow; 2009. https://elibrary.ru/nkranl (in Russian)
- Antonova A.A., Lebedev A.V., Ozhmegova E.N., Shlykova A.V., Lapavok I.A., Kuznetsova A.I. Variability of non-structural proteins of HIV-1 sub-subtype A6 (retroviridae: orthoretrovirinae: lentivirus: human immunodeficiency Virus-1, sub-subtype A6) variants circulating in different regions of the Russian Federation. Voprosy virusologii. 2024; 69(5): 470–80. https://doi.org/10.36233/0507-4088-262 https://elibrary.ru/wbbkuq (in Russian)
- Murzakova A., Kireev D., Baryshev P., Lopatukhin A., Serova E., Shemshura A., et al. Molecular epidemiology of HIV-1 subtype G in the Russian Federation. Viruses. 2019; 11(4): 348. https://doi.org/10.3390/v11040348
- Shchemelev A.N., Semenov A.V., Ostankova Yu.V., Naidenova E.V., Zueva E.B., Valutite D.E., et al. Genetic diversity of the human immunodeficiency virus (HIV-1) in the Kaliningrad region. Voprosy virusologii. 2022; 67(4): 310–21. https://elibrary.ru/bkswno (in Russian)
- Makinson A., Masquelier B., Taieb A., Peytavin G., Waldner-Combernoux A., Collin G., et al. Presence of numerous stop codons in HIV-1 reverse transcriptase proviral DNA sequences from patients with virological response to HAART. AIDS. 2006; 20(9): 1327–9. https://doi.org/10.1097/01.aids.0000232242.51286.7b
- Alidjinou E.K., Deldalle J., Robineau O., Hallaert C., Meybeck A., Huleux T., et al. Routine drug resistance testing in proviral HIV-1 DNA: Prevalence of stop codons and hypermutation, and associated factors. J. Med. Virol. 2019; 91(9): 1684–7. https://doi.org/10.1002/jmv.25474
- Bobkova M.R. Defective HIV proviruses: possible involvement in the HIV infection pathogenesis. Voprosy virusologii. 2024; 69(5): 399–414. https://doi.org/10.36233/0507-4088-261 https://elibrary.ru/pselci (in Russia)
- Rossenkhan R., Novitsky V., Sebunya T.K., Musonda R., Gashe B.A., Essex M. Viral diversity and diversification of major non-structural genes vif, vpr, vpu, tat exon 1 and rev exon 1 during primary HIV-1 subtype C infection. PLoS One. 2012; 7(5): e35491. https://doi.org/10.1371/journal.pone.0035491
- Shen C., Gupta P., Wu H., Chen X., Huang X., Zhou Y., et al. Molecular characterization of the HIV type 1 vpr gene in infected Chinese former blood/plasma donors at different stages of diseases. AIDS Res. Hum. Retroviruses. 2008; 24(4): 661–6. https://doi.org/10.1089/aid.2007.0270
Supplementary files