The results of the detection of specific IgG antibodies to Ebola virus (Filoviridae: Orthoebolavirus) in residents of the Republic of Guinea after the end of the epidemic
- Authors: Naidenova E.V.1, Kartashov M.Y.2, Shulgina I.S.2, Pyankov S.A.2, Kulagin M.A.1, Bah M.B.3, Nourdine I.4, N’Fally M.4, Konomou V.5, Traore M.S.3, Boumbaly S.4, Kutyrev V.V.1
-
Affiliations:
- Russian Research Anti-Plague Institute «Microbe» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
- State Scientific Center of Virology and Biotechnology «Vector» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
- Research Institute of Applied Biology of Guinea
- Virological Research Center, Laboratory of Viral Hemorrhagic Fevers of Guinea
- Scientific Clinical and Diagnostic Center of Epidemiology and Microbiology
- Issue: Vol 70, No 2 (2025)
- Pages: 154-163
- Section: ORIGINAL RESEARCH
- URL: https://virusjour.crie.ru/jour/article/view/16710
- DOI: https://doi.org/10.36233/0507-4088-288
- EDN: https://elibrary.ru/ukfplk
- ID: 16710
Cite item
Abstract
Introduction. In 2014–2016, an epidemic of Ebola virus disease (EVD) was registered in Guinea. In 2021, EVD cases were repeated in the region. The importance of studying the duration of post-infection immunity to the Ebola virus in the body of convalescents is due to the fact that after the end of the epidemic they can be the main sources of infection. One of the indicators of the pathogen circulation in a certain area is the detection of specific IgG antibodies in the blood sera of the inhabitants.
The aim of the study is to identify IgG immunoglobulins to Ebola virus in the blood sera of reconvalescents and practically healthy residents of the Republic of Guinea after the end of the epidemic.
Materials and methods. The ELISA method was used to test the blood sera of 9 patients treated at the NKDCEM hospital (Kindia), collected after the end of the disease and up to 72 months after recovery, and 3939 blood serum samples from practically healthy residents of Guinea.
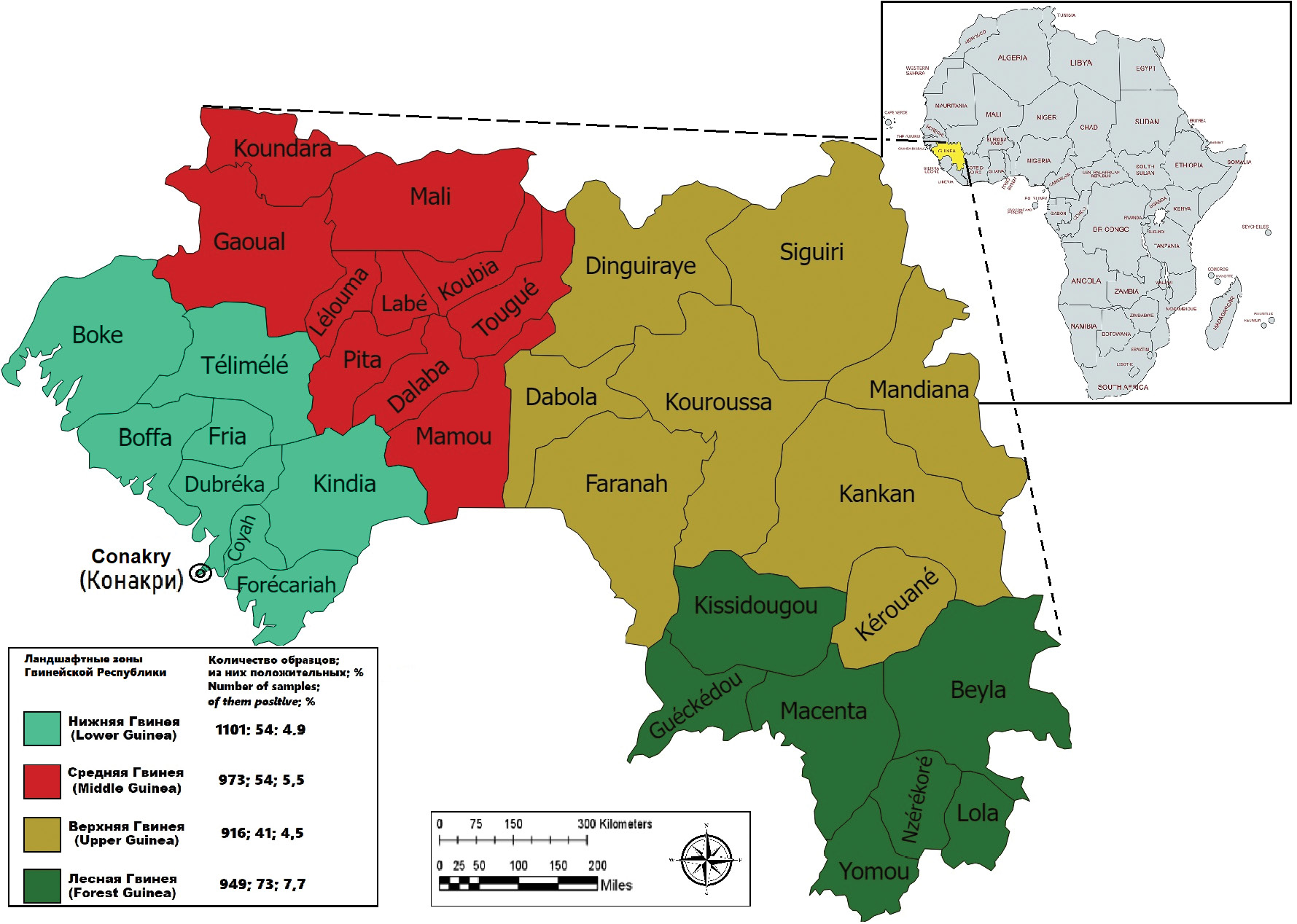
Results. IgG antibodies in the blood sera of reconvalescents a month after recovery were detected in a titer of up to 1 : 800. By 12 months, the antibody level decreased to 1 : 100 and remained at this level for up to 48 months. After 6 years of observation, no antibodies were registered. Among the 3939 blood samples from healthy residents, IgG immunoglobulins to the Ebola virus were detected in 5.6%. Most of the positive samples were collected in Forest Guinea (7.7%), and a smaller part in Upper Guinea (4.5%). The maximum percentage of positive samples was detected in people over 70 years of age (12.3%).
Conclusion. In our case, it was shown that a high level of post-infection immunity in the blood sera of patients with EVD persists for the first 6 months, this corresponds to the data obtained by other authors, and does not exclude the possibility of re-infection. The highest level of the seroprevalence is registered in Forest Guinea. This indicates the active circulation of the pathogen and the constant contact of the inhabitants of the region with it, which leads to epidemiological complications.
Full Text
Introduction
Ebola virus disease (EVD), or Ebola fever, has been known since 1976, when the first documented cases were reported on the border of southern Sudan and northern Zaire (now the Democratic Republic of Congo (DRC)). Over the following decades, periodic outbreaks were recorded in Central African countries (DRC, Sudan, Gabon, Uganda). The total number of patients in those years amounted to 2433 people, the lethality averaged 65% [1, 2].
The situation changed dramatically in December 2013, when EVD rapidly spread to 3 countries in West Africa (Guinea, Liberia, Sierra Leone), where there had been no previous cases. By 2014, the outbreak had reached epidemic proportions, which only ended in June 2016. The total number of patients identified during these events was about 28,000, more than 11,000 of whom died. Molecular genetic studies revealed that the Ebola virus (Filoviridae: Orthoebolavirus, Orthoebolavirus zairense), which caused the international emergency, came from Central Africa, but the route of entry has not yet been determined1 [2].
In early 2021, the government of the Republic of Guinea announced a new outbreak of EVD recorded in the territory of the country, when 16 cases were confirmed by laboratory methods, 12 of which were fatal [3, 4]. When the genetic sequences of the Ebola virus isolated during these events were examined, their profile was found to be almost identical to the strains detected in 2013–2016 and to be in the same phylogenetic cluster with them. These data made it possible to make an assumption about the long-term persistence of the virus in the organism of people who had undergone EVD [5].
In connection with the above-mentioned, the question of the duration of the obtained immunity in reconvalescents is relevant. The need to determine the circulation time of specific immunoglobulin class G (IgG) to Ebola virus in the organism of reconvalescents is emphasized by the fact that after the end of the epidemic, these persons may be the main sources of infection, retaining the ability to transmit the pathogen with breast milk or seminal fluid [6–8]. There is also evidence of re-infection with Ebola virus in two patients in Congo. The infection occurred 11 and 5 months after the initial illness, respectively. The viral meningoencephalitis that developed in both patients was fatal. It has been suggested that viable virus may have persisted in the central nervous system of the reconvalescents. In both cases, specific IgG antibodies were detected in serum and cerebrospinal fluid in the first few days after the onset of clinical signs of infection [9]. High concentrations of IgG antibodies to Ebola virus have been found in the sera of newborns in Liberia born to mothers who have had infection [10].
Studies aimed at investigating the duration of persistence of specific antibodies in the sera of EVD survivors have been conducted in different years. In an enzyme-linked immunosorbent assay (ELISA) study of 29 residents of Kikiwit Province (DRC) who survived the 1995–1996 outbreak, it was found that antibodies to the pathogen persisted for up to 749 days (observation period) in some cases [11]. According to experimental data obtained using a microarray, stable levels of specific IgG antibodies to viral proteins GP, NP and VP40 in the sera of blood sera of EVD survivors persisted for 14 years after infection, but cross-reacted with other filoviruses used in the study [12]. Some studies have shown that some of the antibodies in the blood serum of EVD patients can neutralize the live virus 40 years after infection [13]. In a mass screening of sera from survivors of EVD in the Republic of Guinea using the author’s Luminex-based multiplex assay, it was shown that the presence of IgG antibodies to one or more Ebola virus antigens persisted up to 60 months of follow-up, although antibodies were not detected in approximately 25% of subjects. This does not exclude a possible decline in herd immunity and the occurrence of new outbreaks in the same region [14]. However, no clear data on the level of specific antibodies capable of providing protection against subsequent re-infections in reconvalescents could be found in the available sources.
It is also known that one of the indicators of pathogen circulation in a certain area is the detection of IgG antibodies to the pathogen in the blood sera of persons living in the area. At different periods of time, studies have been conducted to study the herd immunity to the Ebola virus in different African countries.
For example, in the period from 1985 to 1987, a mass survey of residents of Central African countries (Gabon, Cameroon, Congo, Central African Republic (CAR), Chad, and Equatorial Guinea) to detect specific IgG antibodies to viral hemorrhagic fever pathogens relevant to the region revealed antibodies to Ebola virus in an average of 12.4% of samples [15].
In the following years, several other studies were conducted in populations from countries where EVD outbreaks had occurred (DRC and Gabon) and where no cases had been reported at that time (Cameroon, CAR, and Mali) [16–21].
In a study of serum samples from urban and rural residents of Kikwit Province (DRC), the proportion of individuals with specific antibodies to Ebola virus was 2.2 and 9.3%, respectively [17]. A large-scale serologic survey of a rural population in the Gabonese Republic was also conducted using ELISA, and specific IgG antibodies were detected in 15.3% of cases. The level of the immune layer was significantly higher in forest dwellers (19.4%) than in savannah dwellers [18].
Other authors tested the presence of antibodies to Ebola virus in 1517 practically healthy people in 5 regions of Cameroon using indirect immunofluorescence. A positive result was obtained in 9.7% of cases, confirming the circulation of the virus in the region in the absence of clinical cases. The highest rates were reported among Pygmy tribes living in the rainforest [20]. Epidemiologic monitoring in CAR also showed that Pygmies have a significantly higher Ebola virus seroprevalence (7.02%) than other ethnic groups (4.2%) [20]. Studies in Mali indicate that the population in the southern part of the country bordering Guinea and Côte d’Ivoire is also in contact with filoviruses (6.1% of the total number of samples tested) [21].
The first studies of the EVD pathogen seroprevalence in the population of some regions of Guinea were initiated in 1982 at the Soviet-Guinean Virology and Microbiology Laboratory. Specific IgG antibodies to Ebola virus were detected in 8% of local residents at that time, which confirmed the possible circulation of the pathogen in the area [22, 23]. In the 1990s, the research was discontinued due to the closure of the laboratory, and the question of the spread of Ebola virus in different regions of Guinea remained unstudied.
After the end of the EVD epidemic on the territory of the Republic of Guinea, in the elimination of which Russian specialists also participated, the Government of the Russian Federation decided to open the Russian-Guinean Research Center for Epidemiology and Prevention of Infectious Diseases (hereinafter referred to as the Center), which operates at the Institute of Applied Biology of Guinea in Kindia (Guinea Republic) and is authorized to work with group 3 and 4 pathogenic biological agents [24]. Studies on the detection of specific antibodies to Ebola virus were continued on the basis of the Center’s laboratory.
The aim of this study was to detect specific IgG antibodies to Ebola virus in the blood sera of reconvalescents and practically healthy residents of the Republic of Guinea after the end of the EVD epidemic.
Materials and methods
Samples of clinical and biological material were collected at the Center’s laboratory by Russian and Guinean specialists with special training and experience in working with particularly dangerous viruses, guided by the requirements of sanitary rules and regulations SanPiN 3.3686-21 «Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases» and in-force documents in the territory of the Republic of Guinea.
Blood sera of reconvalescents and practically healthy people were obtained in regional hospitals of the Republic of Guinea by local specialists. Blood was collected in the morning hours on an empty stomach from the ulnar vein in the amount of 5–10 mL into a disposable sterile vacuum tube with clot activator for immunoserological studies and with 3.8% sodium citrate for molecular genetic studies. Subsequently, the samples were delivered to the Center laboratory with the according biological safety and temperature conditions.
In order to study the duration and level of humoral immunity following EVD, clinical material was collected from 9 patients (6 men and 3 women) at the hospital of the Scientific Clinical and Diagnostic Center for Epidemiology and Microbiology (SCDCEM) (Kindia, Republic of Guinea) [24] who were treated in May–July 2015. The study subjects with a confirmed diagnosis of EVD received only maintenance therapy, no experimental drugs or immune sera were used during the acute phase of infection. Blood samples were collected after patients were discharged in the absence of clinical manifestations of the disease and two times (48 hours apart) negative results of reverse transcription polymerase chain reaction (RT-PCR), and then 1, 3, 6, 12, 24, 24, 36, 36, 48, 60, 72 months (approximately at the same time points, ± 5 days apart) after the onset of the disease (Table 1). A total of 88 blood samples were examined during follow-up, as two participants dropped out at the last stage of the study.
Table 1. Detection of specific IgG antibodies to Ebola virus in the blood sera of patients at the SCDCEM hospital at different times from the onset of the disease
Таблица 1. Выявление специфических антител класса IgG к вирусу Эбола в сыворотках крови пациентов госпиталя НКДЦЭМ в разные сроки от начала заболевания
Gender Пол | Age (at the time of the onset of the disease), years Возраст (на момент начала заболевания), лет | Antibody detection results depending on the time of observation (months ± 5 days) Результаты выявления антител в зависимости от сроков наблюдения (месяцы ± 5 сут) | |||||||||
0* | 1 | 3 | 6 | 12 | 24 | 36 | 48 | 60 | 72 | ||
М | 33 | 1 : 800 | 1 : 400 | 1 : 200 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | n.i. / н.и. |
М | 28 | 1 : 400 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 0 | 0 | 0 |
М | 45 | 1 : 400 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 |
М | 25 | 1 : 400 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 |
М | 30 | 1 : 800 | 1 : 800 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 |
М | 26 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | n.i. / н.и. |
F / Ж | 30 | 1 : 800 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 |
F / Ж | 22 | 1 : 1600 | 1 : 1600 | 1 : 800 | 1 : 400 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 |
F / Ж | 49 | 1 : 400 | 1 : 400 | 1 : 400 | 1 : 200 | 1 : 100 | 1 : 100 | 1 : 100 | 1 : 100 | 0 | 0 |
Note. * ‒ at discharge. All patients diagnosed with EVD who participated in the study were treated at the hospital in May–July 2015; n.t. – not tested.
Примечание. * ‒ при выписке. Все пациенты с диагнозом БВВЭ, принимавшие участие в исследовании, проходили лечение в госпитале в мае–июле 2015 г.; н.и. – не исследовали.
A panel of 3939 sera from individuals living in all 4 landscape-geographic zones of the country, of whom 1953 (49.6%) were women and 1986 (50.4%) were men, was prepared to determine the level of herd immunity to the EVD pathogen among the population of the Republic of Guinea (Table 2). The material for the study was collected between 2022 and 2024, i.e., after the end of the epidemic.
Table 2. Detection of specific IgG class antibodies to the Ebola virus in the blood sera of practically healthy residents of the Republic of Guinea
Таблица 2. Выявление специфических антител класса IgG к вирусу Эбола в сыворотках крови практически здоровых жителей Гвинейской Республики
Age group (years old) Возрастная группа (лет) | Number of samples; of them positive; % (95% CI) Количество образцов; из них положительных; % (95% ДИ) | ||
total number of samples общее количество образцов | men мужчины | women женщины | |
Lower (Maritime) Guinea / Нижняя (Приморская) Гвинея | |||
< 10 | 71; 0; 0 (0–5.1) | 38; 0; 0 (0–9.1) | 33; 0; 0 (0–10.4) |
10–20 | 111; 2; 1.8 (0.5–6.3) | 51; 1; 1.9 (0.4–10.3) | 60; 1; 1.7 (0.3–8.7) |
20–30 | 246; 8; 3.2 (1.7–6.3) | 133; 5; 3.8 (1.6–8.5) | 113; 3; 2.6 (0.9–7.5) |
30–40 | 242; 15; 6.2 (3.8–9.9) | 112; 9; 8.0 (4.3–14.5) | 130; 6; 4.6 (2.1–9.7) |
40–50 | 229; 14; 6.1 (3.7–10.0) | 98;7; 7.1 (3.5–14.0) | 131; 7; 5.3 (2.6–10.6) |
50–60 | 122; 8; 6.6 (3.4–12.4) | 63; 4; 6.3 (2.5–15.2) | 59; 4; 6.8 (2.7–16.1) |
> 60 | 80; 7; 8.7 (4.3–16.9) | 33; 3; 9.1 (3.1–23.5) | 47; 4; 8.5 (3.4–19.9) |
Total | Всего | 1101; 54; 4.9 (3.8–6.3) | 528; 29; 5.5 (3.8–7.8) | 573; 25; 4.7 (3.2–6.8) |
Middle Guinea | Средняя Гвинея | |||
< 10 | 57; 4; 7.0 (2.7–16.7) | 24; 2 ;8.3 (2.3–25.8) | 33; 2; 6.1 (1.7–19.6) |
10–20 | 123; 9; 7.3 (3.9–13.3) | 57; 5; 8.7 (3.8–18.9) | 66; 4; 6.1 (2.4–14.5) |
20–30 | 226; 10; 4.4 (2.4–7.9) | 101; 5; 4.9 (2.1–11.0) | 125; 5; 4.0 (1.7–9.0) |
30–40 | 239; 10 4.2 (2.3–7.5) | 110; 6; 5.4 (2.5–11.4) | 129; 4; 3.1 (1.2–7.7) |
40–50 | 165; 7; 4.5 (2.2–8.9) | 87; 5; 5.7 (2.5–12.7) | 78; 2; 2.6 (0.7–8.8) |
50–60 | 101; 7; 6.9 (3.4–13.6) | 46; 4; 8.7 (3.4–20.3) | 55; 3; 5.4 (1.9–14.8) |
> 60 | 62; 7; 11.3 (5.6–21.5) | 37; 4; 10.8 (4.3–24.7) | 25; 3; 12.0 (4.2–29.9) |
Total | Всего | 973; 54; 5.5 (4.3–7.2) | 462; 31; 6.7 (4.7–9.3) | 511; 23; 4.5 (3.0–6.7) |
Upper Guinea | Верхняя Гвинея | |||
< 10 | 49; 0; 0 (0–7.2) | 28; 0; 0 (0–12.1) | 21; 0; 0 (0–15.5) |
10–20 | 128; 0; 0 (0–2.9) | 68; 0; 0 (0–5.3) | 60; 0; 0 (0–6.0) |
20–30 | 199; 7; 3.5 (1.7–7.1) | 102; 3; 2.9 (1.1–8.2) | 97; 4; 4.1 (1.6–10.1) |
30–40 | 190; 8; 4.2 (2.1–8.1) | 100; 5; 5.0 (2.1–11.2) | 90; 3; 3.3 (1.1–9.3) |
40–50 | 188; 11; 5.8 (3.3–10.2) | 104; 6; 5.7 (2.7–12.0) | 84; 5; 5.9 (2.6–13.1) |
50–60 | 90; 9; 10.0 (5.3–17.9) | 51; 4; 7.8 (3.1–18.5) | 39; 5; 12.8 (5.6–26.7) |
> 60 | 72; 6; 8.3 (3.9–17.0) | 39; 3; 7.7 (2.6–20.3) | 33; 3; 9.1 (3.1–23.5) |
Total | Всего | 916; 41; 4.5 (3.3–6.0) | 492; 21; 7.2 (2.8–6.4) | 424; 20; 4.7 (3.1–7.1) |
Forest Guinea | Лесная Гвинея | |||
< 10 | 51; 5; 9.8 (4.3–20.9) | 28; 2; 7.1 (1.9–22.6) | 23; 3; 13.0 (4.5–32.1) |
10–20 | 200; 7; 3.5 (1.7–7.0) | 107; 4; 3.7 (1.4–9.2) | 93; 3; 3.2 (1.1–9.0) |
20–30 | 239; 12; 5.0 (2.9–8.6) | 129; 6; 4.6 (2.1–9.7) | 110; 6; 5.4 (2.5–11.4) |
30–40 | 190; 10; 5.3 (2.9–9.4) | 95; 6; 6.3 (2.9–13.1) | 95; 4; 4.2 (1.6–10.3) |
40–50 | 72; 8; 11.1 (5.7–20.4) | 41; 4; 9.7 (3.8–22.5) | 31; 4; 12.9 (5.1–28.8) |
50–60 | 126; 16; 12.7 (7.9–19.6) | 68; 9; 13.2 (7.1–23.3) | 58; 7; 12.0 (5.9–22.8) |
> 60 | 71; 15; 21.1 (13.2–31.9) | 36; 7; 19.4 (9.7–35.0) | 35; 8; 22.8 (12.1–39.0) |
Total | Всего | 949; 73; 7.7 (6.2–9.5) | 504; 38; 9.5 (5.5–10.2) | 445; 35; 7.8 (5.7–10.7) |
Total by country | Общее по стране | |||
< 10 | 228; 9; 3.9 (2.1–7.3) | 118; 4; 3.4 (1.3–8.4) | 110; 5 4.5 (1.9–10.2) |
10–20 | 562; 18; 3.2 (2.0–5.0) | 283; 13; 4.6 (2.7–7.7) | 279; 5; 1.8 (0.8–4.1) |
20–30 | 910; 37; 4.1 (2.9–5.5) | 465; 19; 4.1 (2.6–6.3) | 445; 18; 4.0 (2.6–6.3) |
30–40 | 861; 43; 4.9 (3.7–6.7) | 417; 24; 5.7 (3.9–8.4) | 444; 19; 4.3 (2.8–6.6) |
40–50 | 654; 40; 6.1 (4.5–8.2) | 330;22; 6.7 (4.4–9.8) | 324; 18; 5.6 (3.5–8.6) |
50–60 | 439; 40; 9.1 (6.7–12.2) | 228; 21; 9.2 (6.1–13.7) | 211; 19; 9.0 (5.8–13.6) |
> 60 | 285; 35; 12.3 (8.9–16.6) | 145; 17; 11.7 (7.4–17.9) | 140; 18; 12.8 (8.3–19.4) |
Total | Всего | 3939; 222; 5.6 (4.9–6.4) | 1986; 119; 6.0 (5.0–7.1) | 1953;103; 5.3 (4.4–6.4) |
Before further analysis, all samples were tested for Ebola virus RNA using RT-PCR with the AmpliSens EBOV Zaire-FL reagent kit (Central Research Institute of Epidemiology, Russia). In all cases a negative result was obtained. To exclude the probability of nonspecific reactions in experimental work, sera were also examined by immunochromatographic analysis for detection of malaria plasmodium antigens using the SDBIOLINE Malaria Ag P.f./Pan diagnostic kit (Standart Diagnostics, Inc., Republic of Korea). Samples containing antigens of malaria pathogens were excluded from subsequent studies.
Sera obtained from convalescent patients were studied in dilutions from 1 : 100 to 1 : 3200, from practically healthy individuals – in titer 1 : 100. The studies were performed by ELISA to detect specific IgG antibodies to Ebola virus using Vector ELISA Ebola-AT Screen reagent kit (RU No. RZN 2015/3458; State Research Center Vector, Russian Federation). The diagnostic specificity and sensitivity of the assay were evaluated during the EVD epidemic in the Republic of Guinea. Subsequently, after passing all stages of clinical and laboratory studies, the kit was officially registered [25].
The described scientific work was approved by the decision of the Ethical Committee of Guinea (Minutes No. 129/CNERS/16 of 31.08.2015). The material was collected after adult patients signed informed consent, and in minors – after authorization of parents or legal representatives. Practically healthy individuals who participated in the study additionally confirmed that they were not infected with Ebola virus in 2014–2016, but did not exclude contact with wild and domestic animals.
Results
As a result of the work, specific IgG antibodies Ebola virus in sera of hospital patients 1 month after the end of the disease were detected in titers from 1 : 200 to 1 : 800. By 12 months, antibody levels in all samples decreased to a titer of 1 : 100 and persisted until 48 months, and after 6 years (72 months) of follow-up, negative results were obtained in all cases (Table 1).
When 3939 blood samples from practically healthy Guinea residents were analyzed, specific IgG antibodies to Ebola virus were detected in 222 cases, representing 5.6% (95% CI 4.9–6.4%). Of the total positive samples, 119 (53.6%) belonged to males and 103 (46.4%) to females. There was no significant dependence of the level of immunity on the gender of the examined individuals. The maximum percentage of positive samples was found in the age group of people over 70 years old (12.3% (95% CI 8.9–16.6%)), and the minimum percentage was found in residents aged 10–20 years old (3.2% (95% CI 2.0–5.0%)).
The majority of positive samples were collected in Forest Guinea (7.7% (95% CI 6.2–9.5%)), where an outbreak of EVD occurred in February–April 2021 [3], and a smaller proportion in Upper Guinea (4.5%) (Table 2, Figure). Immunity to the EVD pathogen for residents of Middle and Lower (Maritime) Guinea was recorded at 5.5% (95% CI 4.3–7.2%) and 4.9% (95% CI 3.8–6.3%), respectively.
Figure. The results of the detection of IgG antibodies to Ebola virus in residents of various landscape-geographical zones of Guinea.
Рисунок. Результаты выявления антител класса IgG к вирусу Эбола у жителей различных ландшафтно-географических зон Гвинеи.
Discussion
Thus, the present study showed that a high level of post-infection immunity in humans after a EVD infection (i.e., when the serum titer exceeds 1 : 100) persists for a short period of time (during the first 6 months), which is consistent with the data obtained by other authors [14], but does not exclude the possibility of re-infection in the future. And, of course, it would be interesting to study the changes in cellular immunity in such studies, which is not always possible under the existing conditions.
Current data on the detection of specific IgG antibodies to the EVD pathogen in practically healthy residents of the Republic of Guinea confirm earlier assumptions about the spread of Ebola virus in the country [22, 23]. It should be noted that the highest level of the immune layer of the population is registered in Forest Guinea, and this is significantly higher than the average statistical values for the country (Table 2, Figure). This territory geographically belongs to the tropical rain forest belt, where, according to the observations of many authors, the circulation of Ebola virus is more active [17]. The population is mostly sedentary, hunting and farming. The lowest level is found in Upper Guinea, most of which belongs to the savannah zone, where nomadic locals graze small and large horned cattle. The heterogeneity of the seroprevalence rates can be explained by the different climatic and ecological conditions of the population, as well as by the different main activities and farming methods, which certainly increases the risk of human contact with the pathogen.
According to several authors, up to 27.1% (95% CI 14.5–39.6%) of Ebola virus infection occurs as an asymptomatic infection [26, 27]. These data suggest a more active circulation of the pathogen and constant contact with it in the inhabitants of Forest Guinea, which can periodically lead to epidemiological complications, as evidenced by the events in 2021.
Conclusion
The data obtained as a result of this work indicate the need to conduct further studies to investigate the possibility of circulation of the EVD pathogen in the territory of the Republic of Guinea, focusing on a specific locality. At the same time, it is necessary to continue regular epizootological monitoring to identify possible warm-blooded Ebola virus carrier species in different landscape and geographical zones of the Republic of Guinea.
1 Health worker Ebola infections in Guinea, Liberia and Sierra Leone Preliminary report. WHO/EVD/SDS/REPORT/2015.1 [Electronic resource]. URL. https://www.who.int/publications/i/item/WHO-EVD-SDS-REPORT-2015.1 (access date 09.11.2024).
About the authors
Ekaterina V. Naidenova
Russian Research Anti-Plague Institute «Microbe» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Author for correspondence.
Email: katim2003@mail.ru
ORCID iD: 0000-0001-6474-3696
PhD (Biology), Leading Researcher at the Department of Diagnostics of Infectious Diseases
Россия, 410005, SaratovMikhail Yu. Kartashov
State Scientific Center of Virology and Biotechnology «Vector» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: Mikkartash@yandex.ru
ORCID iD: 0000-0002-7857-6822
PhD (Biology), Senior Researcher at the Department of Flavivirus Infections
Россия, 630559, Novosibirsk Region, Kol’tsovoIrina S. Shulgina
State Scientific Center of Virology and Biotechnology «Vector» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: Shuigina.is@vector.ru
ORCID iD: 0000-0002-6850-338X
Junior Researcher at the Collection of Microorganisms Department
Россия, 630559, Novosibirsk Region, Kol’tsovoStepan A. Pyankov
State Scientific Center of Virology and Biotechnology «Vector» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: pyanstep@gmail.ru
ORCID iD: 0000-0002-6593-6614
Senior Researcher at the Collection of Microorganisms Department
Россия, 630559, Novosibirsk Region, Kol’tsovoMaxim A. Kulagin
Russian Research Anti-Plague Institute «Microbe» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: rusrapi@mail.ru
ORCID iD: 0000-0002-6423-1460
Junior Researcher at the Department of Diagnostics of Infectious Diseases
Россия, 410005, SaratovMamadou B. Bah
Research Institute of Applied Biology of Guinea
Email: boundoukhourabah@gmail.ru
ORCID iD: 0000-0002-4565-269X
Researcher
Гвинея, KindiaIbrahim Nourdine
Virological Research Center, Laboratory of Viral Hemorrhagic Fevers of Guinea
Email: Ibrahimndin@yahoo.fr
ORCID iD: 0000-0002-2970-9676
PhD (Biology), Researcher, Director
Гвинея, ConakryMagassouba N’Fally
Virological Research Center, Laboratory of Viral Hemorrhagic Fevers of Guinea
Email: cmagassouba01@gmail.com
ORCID iD: 0000-0002-3760-6642
PhD (Biology), Scientific Consultant
Гвинея, ConakryViktor Konomou
Scientific Clinical and Diagnostic Center of Epidemiology and Microbiology
Email: Saet-64@yandex.ru
ORCID iD: 0009-0007-5010-0041
Chief Physician of the Hospital
Гвинея, KindiaMohamed S. Traore
Research Institute of Applied Biology of Guinea
Email: dg@irbag.edu.gn
ORCID iD: 0000-0002-3811-8675
PhD (Biology), Director General
Гвинея, KindiaSanaba Boumbaly
Virological Research Center, Laboratory of Viral Hemorrhagic Fevers of Guinea
Email: drboumbaly@yahoo.fr
ORCID iD: 0000-0002-4506-6033
PhD (Biology), Director
Гвинея, ConakryVladimir V. Kutyrev
Russian Research Anti-Plague Institute «Microbe» of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)
Email: rusrapi@mail.ru
ORCID iD: 0000-0003-3788-3452
Dr Sci (Medicine), Academician of the Russian Academy of Sciences, Professor, Director
Россия, 410005, SaratovReferences
- Gonzalez J.P., Herbreteau V., Morvan J., Leroy E.M. Ebola virus circulation in Africa: a balance between clinical expression and epidemiological silence. Bull. Soc. Pathol. Exot. 2005; 98(3): 210–7.
- Malvy D., McElroy A.K., de Clerck H., Günther S., van Griensven J. Ebola virus disease. Lancet. 2019; 393(10174): 936–48. https://doi.org/10.1016/S0140-6736(18)33132-5
- Keita A.K., Koundouno F.R., Faye M., Düx A., Hinzmann J., Diallo H., et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature. 2021; 597(7877): 539–43. https://doi.org/10.1038/s41586-021-03901-9
- Pare B.C., Camara A.M., Camara A., Kourouma M., Enogo K., Camara M.S., et al. Ebola outbreak in Guinea, 2021: Clinical care of patients with Ebola virus disease. S. Afr. J. Infect. Dis. 2023; 38(1): 454. https://doi.org/10.4102/sajid.v38i1.454
- Kritsky A.A., Keita S., Magassouba N., Krasnov Ya.M., Safronov V.A., Naidenova E.V., et al. Ebola virus disease outbreak in the Republic of Guinea 2021: hypotheses of origin. bioRxiv. Preprint. 2021. https://doi.org/10.1101/2021.04.23.440924
- Lopatin A.A., Naidenova E.V., Safronov V.A., Razdorsky A.S., Utkin D.V., Kas’Yan Zh.A., et al. Studies of Ebola Virus persistence in the Body Fluids of a Patient at Advanced Stages of Convalescence. Problemy osobo opasnykh infektsii. 2015; (3): 73–6. https://doi.org/10.21055/0370-1069-2015-3-73-76 (in Russian)
- Diallo B., Sissoko D., Loman N.J., Bah H.A., Bah H., Worrell M.C., et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin. Infect. Dis. 2016; 63(10): 1353–6. https://doi.org/10.1093/cid/ciw601
- Geisbert T.W. Persistence of Ebola virus RNA in seminal fluid. Lancet Glob. Health. 2017; 5(1): e12–3. https://doi.org/10.1016/S2214-109X(16)30336-9
- Mukadi-Bamuleka D., Edidi-Atani F., Morales-Betoulle M.E., Legand A., Nkuba-Ndaye A., Bulabula-Penge J., et al. Fatal meningoencephalitis associated with Ebola virus persistence in two survivors of Ebola virus disease in the Democratic Republic of the Congo: a case report study. Lancet Microbe. 2024; 5(10): 100905. https://doi.org/10.1016/S2666-5247(24)00137-X
- Fallah M.P., Reilly C., Van Ryn C., Badio M., Camanor S.W., Kaler S.G., et al. Pregnancy, pregnancy outcomes, and infant growth and development after recovery from Ebola virus disease in Liberia: an observational cohort study. Lancet Glob. Health. 2023; 11(7): e1053–60. https://doi.org/10.1016/S2214-109X(23)00210-3
- Baize S., Leroy E.M., Georges-Courbot M.C., Capron M., Lansoud-Soukate J., Debré P., et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 1999; 5(4): 423–6. https://doi.org/10.1038/7422
- Natesan M., Jensen S.M., Keasey S.L., Kamata T., Kuehne A.I., Stonier S.W., et al. Human survivors of disease outbreaks caused by Ebola or Marburg virus exhibit cross-reactive and long-lived antibody responses. Clin. Vaccine Immunol. 2016; 23(8): 717–24. https://doi.org/10.1128/CVI.00107-16
- Rimoin A.W., Lu K., Bramble M.S., Steffen I., Doshi R.H., Hoff N.A., et al. Ebola virus neutralizing antibodies detectable in survivors of the Yambuku, Zaire outbreak 40 years after infection. J. Infect. Dis. 2018; 217(2): 223–31. https://doi.org/10.1093/infdis/jix584
- Diallo M.S.K., Ayouba A., Keita A.K., Thaurignac G., Sow M.S., Kpamou C., et al. Temporal evolution of the humoral antibody response after Ebola virus disease in Guinea: a 60-month observational prospective cohort study. Lancet Microbe. 2021; 2(12): e676–84. https://doi.org/10.1016/S2666-5247(21)00170-1
- Gonzalez J.P., Josse R., Johnson E.D., Merlin M., Georges A.J., Abandja J., et al. Antibody prevalence against haemorrhagic fever viruses in randomized representative Central African populations. Res. Virol. 1989; 140(4): 319–31. https://doi.org/10.1016/s0923-2516(89)80112-8
- Busico K.M., Marshall K.L., Ksiazek T.G., Roels T.H., Fleerackers Y., Feldmann H., et al. Prevalence of IgG antibodies to Ebola virus in individuals during an Ebola outbreak, Democratic Republic of the Congo, 1995. J. Infect. Dis. 1999; 179(Suppl. 1): S102–7. https://doi.org/10.1086/514309
- Rowe A.K., Bertolli J., Khan A.S., Mukunu R., Muyembe-Tamfum J.J., Bressler D., et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J. Infect. Dis. 1999; 179(Suppl. 1): S28–35. https://doi.org/10.1086/514318
- Becquart P., Wauquier N., Mahlakõiv T., Nkoghe D., Padilla C., Souris M., et al. High prevalence of both humoral and cellular immunity to Zaire ebolavirus among rural populations in Gabon. PLoS One. 2010; 5(2): e9126. https://doi.org/10.1371/journal.pone.0009126
- Bouree P., Bergmann J.F. Ebola virus infection in man: a serological and epidemiological survey in the Cameroons. Am. J. Trop. Med. Hyg. 1983; 32(6): 1465–6. https://doi.org/10.4269/ajtmh.1983.32.1465
- Gonzalez J.P., Nakoune E., Slenczka W., Vidal P., Morvan J.M. Ebola and Marburg virus antibody prevalence in selected populations of the Central African Republic. Microbes Infect. 2000; 2(1): 39–44. https://doi.org/10.1016/s1286-4579(00)00287-2
- Bane S., Rosenke K., Maiga O., Feldmann F., Meade-White K., Callison J., et al. Ebola virus IgG seroprevalence in Southern Mali. Emerg. Infect. Dis. 2021; 27(6): 1681–4. https://doi.org/10.3201/eid2706.203510
- Ivanov A.P., Tkachenko E.A., van der Groesen G., Butenko A.M., Konstantinov O.K. Indirect enzyme immunoassay for laboratory diagnosis of hemorrhagic Lassa fever and Ebola. Voprosy virusologii. 1986; 31(2): 186–90. (in Russian)
- Butenko A.M. Retrospective data on the study of Ebola virus in Africa. Epidemiologiya i infektsionnye bolezni. 2015; 20(1): 39–43. https://elibrary.ru/ttmbcv (in Russian)
- Popova A.Yu., Smolensky V.Yu., Naidenova E.V., Shcherbakova S.A., Safronov V.A., Kolomoets E.V., et al. Russia – Guinea: Historical Aspects of Scientific Cooperation in the Fight against Dangerous Infectious Diseases. Problemy osobo opasnykh infektsii. 2024; (3): 6–14. https://doi.org/10.21055/0370-1069-2024-3-6-14 (in Russian)
- P’yankov S.A., P’yankov O.V., Naydenova E.V., Agafonov A.P., Boiro M.Y., Solodkiy V.V., et al. Experience of application of the Elisa method for detection of antibodies to Ebola virus during the Saet team work in the Republic of Guinea. Problemy osobo opasnykh infektsii. 2016; (3): 71–5. https://elibrary.ru/xantbd (in Russian)
- Dean N.E., Halloran M.E., Yang Y., Longini I.M. Transmissibility and pathogenicity of Ebola virus: a systematic review and meta-analysis of household secondary attack rate and asymptomatic infection. Clin. Infect. Dis. 2016; 62(10): 1277–86. https://doi.org/10.1093/cid/ciw114
- Bower H., Glynn J.R. A systematic review and meta-analysis of seroprevalence surveys of ebolavirus infection. Sci. Data. 2017; 4(1): 160133. https://doi.org/10.1038/sdata.2016.133
Supplementary files