Detection of antibodies to the hepatitis E virus in domestic reindeer (Rangifer tarandus) in the Republic of Sakha (Yakutia)
- Authors: Kichatova V.S.1,2,3, Potemkin I.A.1,2,3, Asadi Mobarkhan F.A.1,2, Rumyantseva T.D.4, Semenov S.I.5, Kyuregyan K.K.1,2, Mikhailov M.I.1,2
-
Affiliations:
- Central Research Institute of Epidemiology
- Mechnikov Research Institute of Vaccines and Sera
- Russian Medical Academy of Continuing Professional Education
- Arctic State Agrotechnological University
- North-Eastern Federal University named after. M.K. Ammosov
- Issue: Vol 68, No 6 (2023)
- Pages: 549-556
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16590
- DOI: https://doi.org/10.36233/0507-4088-206
- EDN: https://elibrary.ru/ifzfnu
- ID: 16590
Cite item
Abstract
Introduction. Although domestic pigs and wild boars are the main reservoir of zoonotic hepatitis E virus (HEV) genotypes in temperate countries, the presence of antibodies to HEV (anti-HEV) in the indigenous population of circumpolar territories, i.e. outside the habitat of wild and domestic pigs, indicates the presence of an alternative reservoir of the virus. Reindeer (Rangifer tarandus) may be a potential reservoir for HEV in the polar regions. The purpose of the study was to determine the prevalence of anti-HEV among domestic reindeer in the Republic of Sakha (Yakutia).
Materials and methods. Sera from 497 domestic reindeer from the Oymyakon (n = 425) and Ust-Yansky districts (n = 72) of the Republic of Sakha (Yakutia) were tested for anti-HEV. A commercial ELISA kit DS-ELISA-ANTI-HEV-G (Diagnostic Systems-Stolitsa LLC, Russia) was used for detection of anti-HEV IgG, but a rabbit polyclonal antibody against deer IgG labeled with horseradish peroxidase (KPL, USA) at a dilution of 1 : 100 in phosphate-buffered saline were used instead of the human specific conjugate from the kit.
Results. The average detection rate of anti-HEV in reindeer sera was 15.5% (95% CI: 12.6–19.0%). The detection rate of anti-HEV significantly increased with age, from 3.5% (95% CI: 1.1–9.0%) in calves aged 3–6 months to 25.0% (95% CI: 1.6 –36.5%) in deer aged 2–4 years (p < 0.0001). From this age group, anti-HEV detection rates reached a plateau, not differing significantly between older age groups (p > 0.05). The average anti-HEV detection rate among reindeer 2 years of age and older was 19.0% (95% CI: 15.3–23.4%). There were no statistically significant differences in the frequency of anti-HEV detection between female and male reindeer, both among adult animals and among calves.
Conclusion. The observed anti-HEV detection rates among domestic reindeer in the Republic of Sakha (Yakutia) indicate that infection caused by HEV or an antigenically similar virus is common in these animals. The dynamics of antibody accumulation in the reindeer population indicates that infection apparently occurs during the first two years of life.
Keywords
Full Text
Introduction
Hepatitis E virus (HEV), or Paslahepevirus balayani (Hepeviridae family, Paslahepevirus genus) according to ICTV taxonomy, is an RNA virus that causes acute hepatitis. However, chronic hepatitis develops in immunosuppressed patients [1]. Furthermore, this virus is characterized by tropism not only to hepatocytes, but also to a wide range of other tissues, which causes frequently reported extrahepatic manifestations of the infection, including neurological [2]. HEV causes at least 20 million infections worldwide each year, about 3.3 million of which are accompanied by the development of symptomatic disease, has a mortality rate of 0.2–4.0% and is the most common cause of acute viral hepatitis [3]. Previously, this infection was thought to be relevant to tropical countries with low levels of sanitary welfare, but in recent years, due to increased awareness, surveillance of HEV and diagnostic coverage, it has become clear that HEV infection is widespread in industrialized countries as well [4]. The results of recent studies show that Russia is also characterized by a high prevalence of the HEV infection and the peculiarities of the epidemiology of infection observed in industrialized temperate countries [5].
The epidemiology as well as the pathogenicity of the HEV infection largely depend on the HEV genotype. Currently, there are 8 known HEV genotypes [6]. Genotypes 1 and 2 are strictly anthroponotic, causing outbreaks and sporadic cases in developing countries [7]. Other genotypes of the virus are capable of infecting different mammalian species such as: wild boars (genotypes 3, 5 and 6), domestic pigs (genotypes 3 and 4), deer (genotypes 3 and 4), rabbits (genotype 3ra) and camels (genotypes 7 and 8) [8]. Genotypes 3 and 4 are associated with all non-imported (autochthonous) cases of human infection with HEV in industrialized countries, with domestic pigs recognized as the main source of infection. According to a consolidated expert consensus, HEV ranks 6th among viruses with a high risk of zoonotic transmission, emphasizing its zoonotic potential [9]. Apart from HEV itself, a number of other members of the Hepeviridae family, primarily Rocahepevirus ratti circulating among rats, are also capable of causing disease in humans [10].
Although pigs, domestic and wild, are the main zoonotic reservoir of HEV genotypes 3 and 4 in temperate countries, the presence of anti-HEV antibodies in indigenous populations in circumpolar areas [11], i.e. outside the habitat of wild and domestic pigs, indicates the presence of an alternative reservoir of the virus. Reindeer (Rangifer tarandus) may be a potential reservoir of HEV in circumpolar regions. Reindeer are the main farm animal in the vast circumpolar areas and the main source of meat for the population, including the indigenous population of the North. In addition to pigs, HEV of genotypes 3 and 4 are known to be detected in different European and Asian deer species [12]. Furthermore, Paslahepevirus alci, the virus phylogenetically closest to HEV of all hepeviruses, has been identified in moose [13]. However, despite several reports of anti-HEV detection in reindeer [14–16], the virus itself has not yet been isolated from this animal species.
We have previously described cases of anti-HEV detection in both reindeer herders and domestic reindeer in the Republic of Sakha (Yakutia) [17]. The aim of the present study was to determine the prevalence of anti-HEV antibodies in reindeer in Yakutia on an expanded sample including reindeer of different ages: from reindeer less than one year of age to over 10 years old.
Materials and methods
A total of 497 blood serum samples were collected from domestic reindeer in Oymyakonsky (n = 425) and Ust-Yansky districts (n = 72) of the Republic of Sakha (Yakutia) during the summer-autumn periods of 2022–2023. In the Ust-Yansky district, blood serum samples were collected mainly from reindeer under 4 months of age (n = 60), while in the Oymyakonsky district, samples were collected from animals of all age groups: from reindeer under 6 months of age to reindeer over 10 years of age. The distribution of samples by study region and in the total sample depending on sex and age of animals is shown in Table 1. The sex and exact age of the animal were undetermined for 21 of the samples, but they were obtained from animals 2 years of age and older; therefore, they were taken into account when calculating the average anti-HEV detection rate and the average cut-off index (COI; signal sample/cut-off), but were excluded from further analysis of the distribution of seropositivity rates depending on the sex and age of the animals.
Table 1. Distribution of blood serum samples of reindeer depending on sex and age of animals
Таблица 1. Распределение образцов сыворотки крови северных оленей в зависимости от пола и возраста животных
Age Возраст | Sex Пол | Oymyakonsky District Оймяконский район | Ust-Yansky District Усть-Янский район | Total Всего |
Les than 6 months До 6 мес | Female Самки | 44 | 28 | 72 |
Male Самцы | 9 | 32 | 41 | |
Total Всего | 53 | 60 | 113 | |
2–4 years 2–4 года | Female Самки | 38 | 6 | 44 |
Male Самцы | 20 | 4 | 24 | |
Total Всего | 58 | 10 | 68 | |
5–6 years 5–6 лет | Female Самки | 139 | 1 | 140 |
Male Самцы | 16 | 1 | 17 | |
Total Всего | 155 | 2 | 157 | |
7–8 years 7–8 лет | Female Самки | 89 | 0 | 89 |
Male Самцы | 4 | 0 | 4 | |
Total Всего | 93 | 0 | 93 | |
Over 9 years Старше 9 лет | Female Самки | 41 | 0 | 41 |
Male Самцы | 4 | 0 | 4 | |
Total Всего | 45 | 0 | 45 | |
Undetermined Не установлен | Undetermined Не установлен | 21 | 0 | 21 |
Blood samples were collected as part of routine veterinary control in compliance with institutional and national standards for ethical treatment of animals. The study protocol was approved by the Ethical Committee of the Federal State Autonomous Educational Institution of Higher Education “Ammosov North-Eastern Federal University” (protocol #34 dated 30.03.2022).
Blood samples were collected from the jugular vein of the animal into blood tubes (BD Vacutainer; BD, UK) using a veterinary Bobrov needle (“MIZ-Vorsma”, Russia) for reindeer aged 2 years and older or an injection needle (KDM, KD-FINE 18G × 1.5”, Germany) for reindeer aged up to one year. Serum separation was performed by natural sedimentation for 24 h at 18–20 °C. Serum samples were transferred into sterile polypropylene tubes and stored at 2–8 °C for no more than 2 days, then frozen at −18 to −20 °C and transported, maintaining a cold chain, to the laboratory, where they were stored at −70 °C until testing.
Anti-HEV IgG antibodies were detected using a commercial ELISA reagent kit “DS-ELISA-ANTI- HEV-IgG” (Diagnostic Systems “Stolitsa” LLC, Russia) according to the manufacturer’s protocol, but the rabbit polyclonal antibodies against deer IgG labeled with horseradish peroxidase (KPL, USA) in dilution 1 : 100 in phosphate-salt buffer were used instead of human specific conjugate from the kit. The ELISA kit used is based on recombinant HEV capsid protein antigen, highly conserved in different HEV genotypes, and has a sensitivity of 1000 mIU/mL [18]. Optical density (OD) was measured with a spectrophotometer at a wavelength of 450 nm. The HEV-positive and negative control samples supplied with the kit were used as positive and negative controls in each plate, respectively. Anti-HEV-reactive deer blood serum from a previous study [17] was also used to monitor the reproducibility of results between runs. Samples with OD values exceeding a cut-off value (0.20 plus the mean OD of negative controls) were considered reactive. For each reactive sample, the COI was calculated as the ratio of the sample OD to the cut-off OD value.
Statistical analysis of the results was performed using GraphPad 10.0.2 software (https://www.graphpad.com/). Statistical analysis of the data included: determination of mean values, calculation of 95% confidence interval (95% CI), identification of reliability of differences in mean values of indicators in the compared groups using Fisher’s exact test (for relative indicators) and unpaired Student’s test (for quantitative indicators). Differences were considered as statistically significant at p ≤ 0.05.
Results
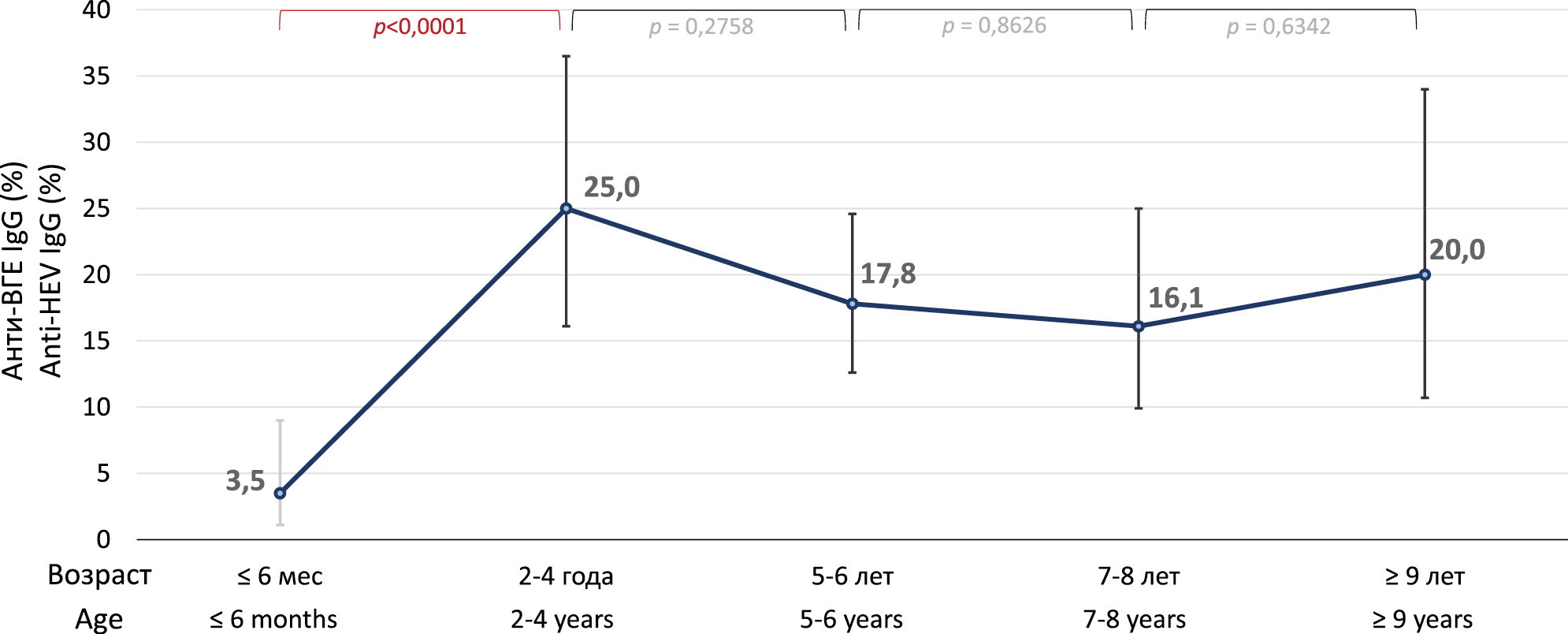
On average, the detection rate of anti-HEV antibodies in reindeer sera was 15.5% (77/497; 95% CI 12.6–19.0%). In samples from reindeer from Oymyakonsky District, the detection rate of anti-HEV was 16.5% (70/425; 95% CI 13.2–20.3%), and in samples from Ust-Yansky District, 9.7% (7/72; 95% CI 4.5–19.0%), and the differences between districts were not statistically significant (p = 0.1618). Therefore, the two samples were combined for further analysis. The results of anti-HEV detection in different age groups of deer are summarized in the figure. The anti-HEV detection rates increased significantly from 3.5% (4/113; 95% CI 1.1–9.0%) in calves aged 3–6 months to 25.0% (17/68; 95% CI 16.1-36.5%) in reindeer aged 2–4 years (p < 0.0001), and reached a plateau from this age onwards, not differing significantly between older age groups (p > 0.05). Overall, the detection rate of anti-HEV among adult reindeer aged 2 years and older was 19.0% (69/363; 95% CI 15.3–23.4%), which was significantly higher than that among calves aged 6 months or less (p = 0.0002).
Figure. Frequency rates of anti-HEV detection in different age groups of reindeer.
Рисунок. Показатели частоты выявления анти-ВГЕ в разных возрастных группах северных оленей.
The frequency of anti-HEV detection depending on the sex of the animals is shown in Table 2. There were no statistically significant differences in the frequency of anti-HEV detection between females and males of reindeer among both adults and calves.
Table 2. Frequency rates of anti-HEV detection in male and female reindeer
Таблица 2. Показатели частоты выявления анти-ВГЕ у самцов и самок северных оленей
Age group Возрастная группа | Anti-HEV, n reactive/N animals examined (%) Анти-ВГЕ, n реактивных/N обследованных животных (%) | p* | |
males самцы | females самки | ||
Calves, 4–6 months Оленята, 4–6 мес | 2/39 (5,1%) | 2/70 (2,9%) | 0,6202 |
Adult reindeer, 2 years and older Взрослые олени, 2 года и старше | 12/37 (32,4%) | 57/257 (22,2%) | 0,3272 |
Note. *p values were obtained when comparing between two groups of reindeer using Fisher’s exact test.
Примечание. *Значения р получены при сравнении между двумя группами животных с использованием критерия Фишера.
The average COI value of anti-HEV reactive reindeer serum samples (±SD) was 4.03 ± 3.54; 57.1% (44/77) of anti-HEV reactive samples had COI ≥ 2. The mean COI values of reactive sera depending on the age of the animals are shown in Table 3. There were no statistically significant differences between the average COI values depending on the age of the animals.
Table 3. Mean values of the cut-off index of anti-HEV reactive sera of reindeer in different age groups of animals
Таблица 3. Средние значения коэффициента позитивности реактивных по анти-ВГЕ сывороток северных оленей в разных возрастных группах животных
Age group Возрастная группа | Average COI value ± SD Среднее значение КП ± SD | p* |
4–6 months 4–6 мес | 3,77 ± 3,23 | |
2–4 years 2–4 года | 3,98 ± 2,85 | 0,8959 |
5–6 years 5–6 лет | 4,41 ± 4,32 | 0,7775 |
7–8 years 7–8 лет | 3,81 ± 3,51 | 0, 9844 |
9 years and older 9 лет и старше | 3,71 ± 2,86 | 0, 9747 |
All age groups Все возрастные группы | 4,03 ± 3,54 | 0,8822 |
Note. *p values when compared to the data for the reindeer calves using unpaired Student’s test.
Примечание. *Значения р при сравнении с данными для группы оленят с использованием непарного критерия Стьюдента.
Discussion
The results obtained indicate that infection caused by HEV or an antigenically similar virus is widespread among domestic reindeer in Yakutia. The detection of anti-HEV in reindeer in the present study does not necessarily indicate the circulation of Paslahepevirus balayani among these animals, since cross-reactivity has been demonstrated for capsid proteins of different representatives of the Hepeviridae family [19–21]. Antibodies to the capsid protein of HEV are usually the target of serologic tests used for the diagnosis of hepatitis E, including the test system used in the present study. Thus, serum reactivity of reindeer in our study may indicate the presence of antibodies to HEV or other HEV-like viruses. Indirect evidence of the specificity of anti-HEV detection in our study is the COI value exceeding the value of 1.5 in 71.4% of reactive samples.
The average frequency of anti-HEV detection in the present study (15.5%) was similar to the ratio of seropositive semi-domesticated reindeer in Norway (15.7%) [16], but substantially higher than in Canada (8.8%) [14]. Comparison with our earlier results of anti-HEV detection in a smaller sample of reindeer from the Oymyakonsky and Anabarsky districts of Yakutia (12.0%; 23/191) [17] demonstrated no significant differences (p = 0.2783). Much like in the studies mentioned above, the ratio of seropositive males and females in the present study was similar. At the same time, we demonstrated for the first time a significant increase in the frequency of anti-HEV detection as the animals matured; compared to 4–6 month-old calves, the prevalence of anti-HEV increased among 2-year-old reindeer and remained at approximately the same level in older age groups. In the reindeer groups investigated in Norway, anti-HEV antibodies were slightly more common in adult reindeer compared with the calves, but these differences were not statistically significant [16], probably due to the small sample sizes in each region of the country investigated.
It should be noted that to this day, HEV has not been systematically searched for in reindeer species. Several studies in which primers specific for Paslahepevirus balayani were used to detect HEV RNA have yielded negative results [14, 17]. No hepevirus sequences were detected either when using a metagenomic approach to analyze blood serum and rectal swabs from wild and domestic reindeer from Fennoscandia and Yakutia [22]. The reasons for failing to detect HEV RNA in these animals could be the significant genetic difference between reindeer HEV and known strains of the Paslahepevirus balayani species if HEV-specific primers were used and the fact that HEV was searched for mainly in adult animals. Among domestic pigs, most cases of HEV infection occur in piglets aged 2–4 months, as they become susceptible to the virus after the disappearance of maternal antibodies [23]. Similarly, most cases of HEV infection in reindeer can also occur at an early age, as indicated by our results of anti-HEV detection in animals of different ages. The revealed dynamics of antibody accumulation in the population of reindeer indicates that infection apparently occurs in the first 2 years of life, after which the animals retain humoral immunity. Thus, it is advisable to further search for sequences of hepeviruses in reindeer among young animals, using metagenomic methods or more universal primers if the polymerase chain reaction (PCR) method is used.
Conclusion
The obtained results of anti-HEV detection among domestic reindeer in the Republic of Sakha (Yakutia) indicate a wide spread of infection caused by HEV or an antigenically similar virus among these animals. The dynamics of anti-HEV accumulation in the population of reindeer indirectly testifies to the peculiarity of the epidemiology of the infection, similar to that observed in pigs - the infection with the virus usually occurs in young animals with subsequent seroconversion and immunity formation. Another similarity observed is the possibility of reindeer calves posing the greatest risk of infection for humans, as they are more likely to have virus replication accompanied by viremia and virus shedding.
About the authors
Vera S. Kichatova
Central Research Institute of Epidemiology; Mechnikov Research Institute of Vaccines and Sera; Russian Medical Academy of Continuing Professional Education
Author for correspondence.
Email: vera_kichatova@mail.ru
ORCID iD: 0000-0002-7838-6965
MD, PhD, Senior Research Fellow, Laboratory of Molecular Epidemiology of Viral Hepatitis of Central Research Institute of Epidemiology; Research Fellow, Laboratory of Viral Hepatitis of I. Mechnikov Research Institute of Vaccines and Sera; Senior Research Fellow, Laboratory of Socially Significant Viral Infections of Russian Medical Academy of Continuing Professional Education, Moscow, Russia
Russian Federation, 111123, Moscow; 105064, Moscow; 125993, MoscowIlya A. Potemkin
Central Research Institute of Epidemiology; Mechnikov Research Institute of Vaccines and Sera; Russian Medical Academy of Continuing Professional Education
Email: axi0ma@mail.ru
ORCID iD: 0000-0001-7559-4219
MD, PhD, Senior Research Fellow, Laboratory of Molecular Epidemiology of Viral Hepatitis of Central Research Institute of Epidemiology; Research Fellow, Laboratory of Viral Hepatitis of I. Mechnikov Research Institute of Vaccines and Sera; Senior Research Fellow, Laboratory of Socially Significant Viral Infections of Russian Medical Academy of Continuing Professional Education, Moscow, Russia
Russian Federation, 111123, Moscow; 105064, Moscow; 125993, MoscowFedor A. Asadi Mobarkhan
Central Research Institute of Epidemiology; Mechnikov Research Institute of Vaccines and Sera
Email: 1amfa@bk.ru
ORCID iD: 0000-0002-1838-8037
Research Fellow, Laboratory of Molecular Epidemiology of Viral Hepatitis of Central Research Institute of Epidemiology; Junior Research Fellow, Laboratory of Viral Hepatitis of I. Mechnikov Research Institute of Vaccines and Sera, Moscow, Russia
Russian Federation, 111123, Moscow; 105064, MoscowTatyana D. Rumyantseva
Arctic State Agrotechnological University
Email: tanya_rum@mail.ru
ORCID iD: 0000-0003-0997-5499
Leading Researcher, Arctic State Agrotechnological University, Yakutsk, Russia
Russian Federation, 677008, Yakutsk, Republic of Sakha (Yakutia)Sergey I. Semenov
North-Eastern Federal University named after. M.K. Ammosov
Email: insemenov@yandex.ru
ORCID iD: 0000-0001-8099-2270
MD, PhD, Leading Researcher, Research Center of the NEFU Medical Institute of the Federal State Autonomous Educational Institution of Higher Education “North-Eastern Federal University named after M.K. Ammosov”, Yakutsk, Russia
Russian Federation, 677010, Yakutsk, Republic of Sakha (Yakutia)Karen K. Kyuregyan
Central Research Institute of Epidemiology; Mechnikov Research Institute of Vaccines and Sera
Email: karen-kyuregyan@yandex.ru
ORCID iD: 0000-0002-3599-117X
Doctor of Biol. Sci., PhD, Professor of Russ. Acad. Sci., Head of Laboratory of Molecular Epidemiology of Viral Hepatitis of Central Research Institute of Epidemiology; Leading Research Fellow, Laboratory of Viral Hepatitis of I. Mechnikov Research Institute of Vaccines and Sera, Moscow, Russia
Russian Federation, 111123, Moscow; 105064, MoscowMikhail I. Mikhailov
Central Research Institute of Epidemiology; Mechnikov Research Institute of Vaccines and Sera
Email: michmich2@yandex.ru
ORCID iD: 0000-0002-6636-6801
MD, PhD, Corr. Member of Russ. Acad. Sci., Chief Research Fellow, Laboratory of Molecular Epidemiology of Viral Hepatitis of Central Research Institute of Epidemiology; Head of Laboratory of Viral Hepatitis of I. Mechnikov Research Institute of Vaccines and Sera; Moscow, Russia
Russian Federation, 111123, Moscow; 105064, MoscowReferences
- Purdy M.A., Drexler J.F., Meng X.J., Norder H., Okamoto H., Van der Poel W.H.M., et al. ICTV virus taxonomy profile: Hepeviridae 2022. J. Gen. Virol. 2022; 103(9). https://doi.org/10.1099/jgv.0.001778
- Pischke S., Hartl J., Pas S.D., Lohse A.W., Jacobs B.C., Van der Eijk A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017; 66(5): 1082–95. https://doi.org/10.1016/j.jhep.2016.11.016.
- WHO. Hepatitis E: Fact Sheet; 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e
- Kamar N., Izopet J., Pavio N., Aggarwal R., Labrique A., Wedemeyer H., et al. Hepatitis E virus infection. Nat. Rev. Dis. Primers. 2017; 3: 17086. https://doi.org/10.1038/nrdp.2017.86
- Mikhailov M.I., Karlsen A.A., Potemkin I.A., Isaeva O.V., Kichatova V.S., Malinnikova E.Y., et al. Geographic and temporal variability of hepatitis E virus circulation in the Russian Federation. Viruses. 2022; 15(1): 37. https://doi.org/10.3390/v15010037
- Smith D.B., Izopet J., Nicot F., Simmonds P., Jameel S., Meng X.J., et al. Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J. Gen. Virol. 2020; 101(7): 692–8. https://doi.org/10.1099/jgv.0.001435
- Nelson K.E., Labrique A.B., Kmush B.L. Epidemiology of genotype 1 and 2 hepatitis E virus infections. Cold Spring Harb. Perspect. Med. 2019; 9(6): a031732. https://doi.org/10.1101/cshperspect.a031732
- Pallerla S.R., Harms D., Johne R., Todt D., Steinmann E., Schemmerer M., et al. Hepatitis E virus infection: circulation, molecular epidemiology, and impact on global health. Pathogens. 2020; 9(10): 856. https://doi.org/10.3390/pathogens9100856
- Grange Z.L., Goldstein T., Johnson C.K., Anthony S., Gilardi K., Daszak P., et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl Acad. Sci. USA. 2021; 118(15): e2002324118. https://doi.org/10.1073/pnas.2002324118
- Reuter G., Boros Á., Pankovics P. Review of hepatitis E virus in rats: evident risk of species Orthohepevirus C to human zoonotic infection and disease. Viruses. 2020; 12(10): 1148. https://doi.org/10.3390/v12101148
- Minuk G.Y., Sun A., Sun D.F., Uhanova J., Nicolle L.E., Larke B., et al. Serological evidence of hepatitis E virus infection in an indigenous North American population. Can. J. Gastroenterol. 2007; 21(7): 439–42. https://doi.org/10.1155/2007/289059
- Di Profio F., Sarchese V., Palombieri A., Fruci P., Lanave G., Robetto S., et al. Current knowledge of Hepatitis E Virus (HEV) epidemiology in ruminants. Pathogens. 2022; 11(10): 1124. https://doi.org/10.3390/pathogens11101124
- Lin J., Karlsson M., Olofson A.S., Belák S., Malmsten J., Dalin A.M., et al. High prevalence of hepatitis e virus in Swedish moose – a phylogenetic characterization and comparison of the virus from different regions. PLoS One. 2015; 10(4): e0122102. https://doi.org/10.1371/journal.pone.0122102
- Weger S., Elkin B., Lindsay R., Bollinger T., Crichton V., Andonov A. Hepatitis E virus seroprevalence in free-ranging deer in Canada. Transbound. Emerg. Dis. 2017; 64(3): 1008–11. https://doi.org/10.1111/tbed.12462
- Sacristán C., Madslien K., Sacristán I., Klevar S., das Neves C.G. Seroprevalence of hepatitis E virus in moose (Alces alces), reindeer (Rangifer tarandus), red deer (Cervus elaphus), roe deer (Capreolus capreolus), and muskoxen (Ovibos moschatus) from Norway. Viruses. 2021; 13(2): 224. https://doi.org/10.3390/v13020224
- Rinaldo C.H., Nymo I.H., Sánchez Romano J., Breines E.M., Murguzur F.J.A., Tryland M. Serological evidence of hepatitis E virus infection in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus) in Norway. Pathogens. 2021; 10(12): 1542. https://doi.org/10.3390/pathogens10121542
- Slukinova O.S., Kyuregyan K.K., Karlsen A.A., Potemkin I.A., Kichatova V.S., Semenov S.I., et al. Serological evidence of hepatitis E virus circulation among reindeer and reindeer herders. Vector. Borne Zoonotic Dis. 2021; 21(7): 546–51. https://doi.org/10.1089/vbz.2020.2727
- Kodani M., Kamili N.A., Tejada-Strop A., Poe A., Denniston M.M., Drobeniuc J., et al. Variability in the performance characteristics of IgG anti-HEV assays and its impact on reliability of seroprevalence rates of hepatitis E. J. Med. Virol. 2017; 89(6): 1055–61. https://doi.org/10.1002/jmv.24741
- Haqshenas G., Huang F.F., Fenaux M., Guenette D.K., Pierson F.W., Larsen C.T., et al. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J. Gen. Virol. 2002; 83(Pt. 9): 2201–9. https://doi.org/10.1099/0022-1317-83-9-2201
- Zhou X., Kataoka M., Liu Z., Takeda N., Wakita T., Li T.C. Characterization of self-assembled virus-like particles of dromedary camel hepatitis E virus generated by recombinant baculoviruses. Virus Res. 2015; 210: 8–17. https://doi.org/10.1016/j.virusres.2015.06.022
- Kubickova B., Schenk J.A., Ramm F., Markuškienė K., Reetz J., Dremsek P., et al. A broadly cross-reactive monoclonal antibody against hepatitis E virus capsid antigen. Appl. Microbiol. Biotechnol. 2021; 105(12): 4957–73. https://doi.org/10.1007/s00253-021-11342-7
- Sánchez Romano J., Omazic A., Leijon M., Hagström Å., Tryland M., Kantanen J., et al. Screening of Eurasian tundra reindeer for viral sequences by next-generation sequencing. Int. J. Environ. Res. Public Health. 2021; 18(12): 6561. https://doi.org/10.3390/ijerph18126561
- Feng R., Zhao C., Li M., Harrison T.J., Qiao Z., Feng Y., et al. Infection dynamics of hepatitis E virus in naturally infected pigs in a Chinese farrow-to-finish farm. Infect. Genet. Evol. 2011; 11(7): 1727–31. https://doi.org/10.1016/j.meegid.2011.07.009
Supplementary files