Properties of influenza viruses that caused epidemic increases in morbidity in Russia and countries of the world during 2022–2023. The effectiveness of vaccine prophylaxis
- Authors: Burtseva E.I.1, Kolobukhina L.V.1, Panova A.D.1, Mukasheva E.A.1, Krasnoslobodtsev K.G.1, Kirillova E.S.1, Breslav N.V.1, Trushakova S.V.1, Komarova I.A.2, Feodoritova E.L.1, Merkulova L.N.1, Khlopova I.N.1, Kruzhkova I.S.1, Ignatieva A.V.1, Krepkaia A.S.1, Komissarov A.B.3, Pochtovyi A.A.1, Kustova D.D.1, Gushchin V.A.1, Tyurin I.N.4, Samkov A.A.4, Antipyat N.А.4
-
Affiliations:
- National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
- Pacific State Medical University of the Ministry of Health of the Russian Federation
- Research institute of influenza named after A.A. Smorodintsev of Ministry of Health
- Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
- Issue: Vol 69, No 1 (2024)
- Pages: 42-55
- Section: ORIGINAL RESEARCHES
- URL: https://virusjour.crie.ru/jour/article/view/16598
- DOI: https://doi.org/10.36233/0507-4088-211
- EDN: https://elibrary.ru/zqtfnx
- ID: 16598
Cite item
Abstract
The purpose of this work was to determine the characteristics of the circulation of various viral respiratory pathogens during the epidemic season 2022–2023 against the background of the ongoing evolutionary variability of SARS-CoV-2.
Materials and methods. The article uses methods used in «traditional» and «hospital» epidemiological surveillance of acute respiratory viral infections.
Results and discussion. The period from October 2022 to September 2023 was characterized by early and high activity of influenza A(H1N1)pdm09 virus, which was replaced by influenza B virus. The antigenic and genetic properties of strains were closely related to influenza vaccines viruses recommended by WHO experts for the current season. The effectiveness of influenza vaccines was confirmed (75.0%). All of the studied influenza A(H1N1)pdm09, A(H3N2) and B epidemic strais retained sensitivity to drugs with antineuraminidase activity. The structure and share of other ARVI pathogens have changed somewhat compared to the previous season: There was a tendency to increase the activity of HAdV and HMPV; almost equivalent activity of HRsV, HRV, HCoV and HBoV; and a decrease in HPIV activity. At the same time, the frequency of other ARVI pathogens did not reach the indicators of the pre-pandemic COVID-19 period. The rationale for updating the composition of influenza vaccines for the countries of the Northern Hemisphere in the 2023–2024 season is given.
Full Text
Introduction
On March 11, 2020, WHO declared the COVID-19 pandemic caused by a new coronavirus, SARS-CoV-2, and on May 5, 2023, announced that the virus no longer posed an emergency public health threat to all countries of the world, but the damage it causes remains a cause for concern [1]. The COVID-19 pandemic has caused serious socio-economic consequences and affected nearly all areas of human activity such as labor, science, education, sports, politics, culture and others. Due to the introduction of quarantine and restrictive measures, the structure of infectious morbidity and, above all, the intensity of influenza epidemics, the share of other respiratory viral pathogens, the involvement of age groups, and the effectiveness of prevention have changed [1–6]. Many researchers have shown that during the years 2020–2022, the intensity of epidemic rises in morbidity was directly related to the activity of SARS-CoV-2 and its new variants (especially Omicron variants), suppression of the activity of other respiratory viral pathogens up to sporadic cases (influenza viruses in the 2020–2021 season), high involvement of the adult population in the epidemic process, high rates of hospitalization, severe forms (SARI), complications and mortality. It should also be noted that existing recommendations and regulations for annual updating of influenza vaccine strain composition using traditional technologies have not been supported for COVID-19 vaccines and require additional clinical trials when changing strains, which is currently a challenge due to the low efficacy of vaccines based on the first Wuhan variant against the new Omicron variants.
The third epidemic season since the beginning of SARS-CoV-2 circulation takes place during 2022–2023. It became obvious that, despite the ongoing evolutionary variability of the new coronavirus, it has, to a certain extent, occupied its niche in the structure of circulating acute respiratory viral infections (ARVI), reducing its virulence and pathogenicity and acquiring the status of a seasonal respiratory infection pathogen. Each of the post-COVID epidemic seasons had its own peculiarities, in this regard, it was of particular interest to assess the development of the influenza epidemic and the incidence of other respiratory pathogens in patients with ARVI in the 2022–2023 epidemic season.
Materials and methods
Collection of data on morbidity and laboratory diagnosis of ARVI pathogens. As part of epidemiological surveillance of influenza and ARVI circulation in the Russian Federation, the Influenza Ecology and Epidemiology Center of the D.I. Ivanovsky Institute of Virology, N.F. Gamaleya National Center of Epidemiology and Microbiology of the Russian Ministry of Health in cooperation with 10 reference bases, represented by territorial departments and the Hygiene and Epidemiology Centers of Rospotrebnadzor in European Russia (Veliky Novgorod, Lipetsk, Vladimir, Yaroslavl, Penza, Cheboksary), the Urals (Orenburg), Siberia (Tomsk) and the Far East (Birobidzhan and Vladivostok), we analyzed incidence rates, hospitalization rates etiologically related to ARVI pathogens in different age groups of the population, as well as the results of laboratory diagnostics. The observation period was from the 40th week (October) of 2022 to the 39th week (September) of 2023.
The analysis of influenza and ARVI in different age groups, isolation of influenza viruses, RT-PCR, HAI, assessment of sensitivity to anti-influenza drugs, and statistical processing of the results obtained have been described in previous studies [5, 6]. Within the framework of traditional surveillance, the volume of studies using laboratory methods amounted to: 39,745 samples for influenza viruses, 35,340 samples for ARVI and 18,873 samples for SARS-CoV-2; within the framework of sentinel surveillance (Moscow only) there were 719 samples for all pathogens.
The efficacy of influenza vaccine prophylaxis was evaluated in hospitalized patients with symptoms of ARVI according to anamnestic data (from the patient’s words) according to WHO recommendations, using the test-negative design approach [7].
Full genomic amplification of influenza A and B viruses was performed according to the previously described methodology [8, 9]. A cDNA library was prepared using the SQK-LSK109 kit (Oxford Nanopore, UK) followed by sequencing on a MinION instrument (Oxford Nanopore, UK). Bioinformatics data processing was performed using guppy ver.6.3.8, porechop ver.0.2.4, nanofilt ver.2.3.0, minimap2 ver.2.24, medaka ver.1.7.2 and bcftools ver.1.13, MEGA 7.0.26 software packages.
The study was conducted with the informed consent of the patients. The protocol of the study was approved by the Ethics Committee of the Infectious Clinical Hospital No. 1 of the Moscow Department of Health (Protocol No. 8 dated 12/28/2022).
Results
During the observation period from October 2022 (week 40) to September 2023 (week 39) in the territories cooperating with the Influenza Ecology and Epidemiology Center, the increase of the epidemic threshold of ARVI morbidity in relation to the average indicator for the Russian Federation (72.6 per 10,000 population) was registered in the period of weeks 46–52 of 2022 (due to the activity of influenza A(H1N1)pdm09 virus), weeks 5–9 of 2023 (increased activity of influenza B virus), weeks 37–39 of 2023 (activity of ARVI of non-influenza etiology). Maximum morbidity in the total population (average value according to the data collected from 10 cities of the Russian Federation) was registered in week 50 of 2022 (157.0 per 10,000), during which the frequency of positive samples for SARS-CoV-2 amounted to 7.3%, ARVI – 15.6% (PCR) and influenza – 28.3%.
It should be noted that the average incidence of ARVI was slightly lower compared to the previous season (71.6 per 10,000 population); at the same time, an increase was registered in children aged 0–2 years (mean 294.4, range 9.2–475.2 and mean 246.8 (7.3–423.2), respectively) and 3–6 years (273.9 (10.5–425.4) and 223.4 (8.3–437.1), respectively). The incidence in school children was comparable to that of the previous year (141.8 (9.4–218.3)); while a decrease was noted in adults (43.6 (19.4–54.3) and 51.1 (35.3–77.8), respectively).
Clinical diagnosis of influenza was made in 9531 patients, 2563 (27.0%) of whom were hospitalized, including patients 0–2 years of age – 529 (20.0%), 3–6 years of age – 417 (16.0%), 7–14 years of age – 404 (15.8%) and 65 years of age and older – 1213 (47.3%). Six cases of influenza infection with fatal outcomes were reported: a man 18 years of age, laboratory confirmed influenza A(H1N1)pdm09 (Penza, December 2022); a woman 73 years of age, diagnosed according to clinical and epidemiologic data (Lipetsk, December 2022); a man 81 years of age and a woman 84 years of age, diagnosed according to clinical and epidemiologic data (Orenburg, December 2022); a woman 33 years of age and a woman 81 years of age, laboratory confirmed influenza A(H1N1)pdm09 (Orenburg, January 2023).
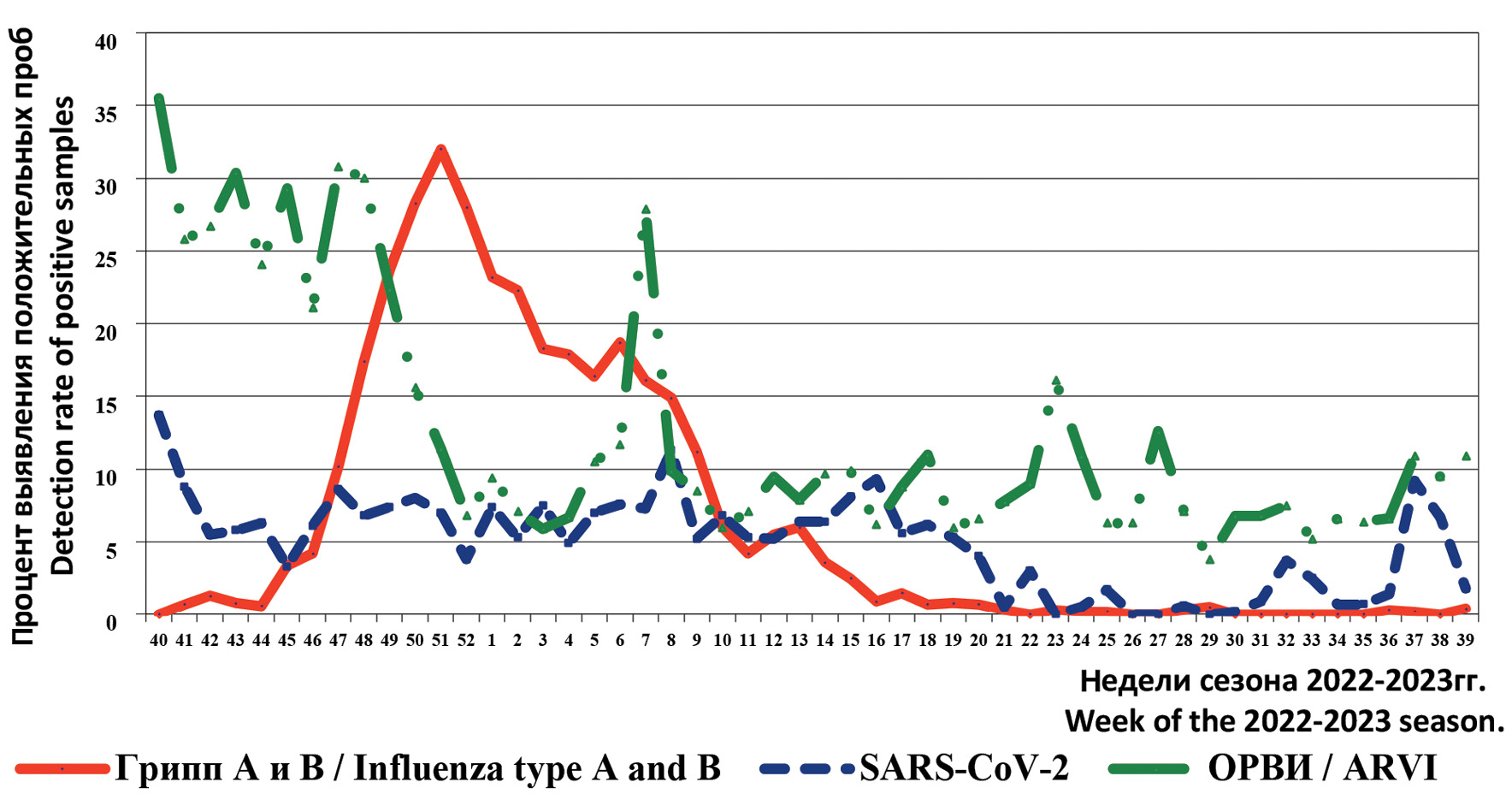
Dynamics of the frequency of positive findings for influenza A and B, SARS-CoV-2 and ARVI viruses (including HPIV, HAdV, HRsV, HRV, HBoV, HMPV, (HCoV) by RT-PCR during the period of October 2022 – September 2023 are presented in Figure 1.
Fig. 1. The frequency of positive samples for influenza, SARS-CoV-2 and ARVI during the epidemic season 2022–2023 in certain territories of the Russian Federation
Рис. 1. Частота выявления положительных проб на грипп, SARS-CoV-2 и ОРВИ в период эпидемического сезона 2022–2023 гг. на отдельных территориях РФ.
The 2022–2023 epidemic season started with a relatively high rate of positive samples for ARVI of non-influenza etiology (40th weeks 2022 – 35.5%). At the same time, during this period the frequency of positive samples for SARS-CoV-2 was significantly lower (13.7%), and influenza viruses were not detected.
Against the background of decreasing activity of non-influenza pathogens and relatively stable frequency of positive SARS-CoV-2 samples (up to 8.6%), a relatively early and sharp increase in the number of positive samples for influenza, mainly of the A(H1N1)pdm09 subtype, was observed in the period of weeks 40–52 of 2022. The first cases of influenza A(H1N1)pdm09 were detected in October 2022 in European Russia cities (2nd week of October), after a certain period of time in the Urals, Siberia and the Far East (second half of November 2022). In December, all cities of the Russian Federation cooperating with the Influenza Ecology and Epidemiology Center registered an increase in influenza A(H1N1)pdm09 virus activity (up to 30.3% of positive samples) and sporadic cases of influenza B (up to 3.0%). These trends correlate with the dynamics of ARVI morbidity rates, which peaked in December 2022.
The activity of influenza viruses began to decrease in early 2023, with detection rates of positive samples above 10% recorded until the beginning of March 2023. An interesting and established fact is the alternating activity of influenza viruses: influenza A(H1N1)pdm09 was replaced by influenza B virus in January 2023, with its highest frequency in the period of one week being significantly lower (14.3%). The last cases were detected in June–July 2023 in Moscow and June–July, as well as September in Orenburg.
The second, lower and short-term wave of increase in the activity of ARVI pathogens was observed in the period of week 7 of 2023 with the maximum frequency of detection of the number of positive samples (27.9%). In contrast to the previous and subsequent weeks, a high frequency of positive samples for HRsV (9.9%) and HRV (6.5%) was observed in Orenburg and Vladivostok.
Since the 20th week of 2023, against the background of decreased activity of influenza and SARS-CoV-2 viruses, a relatively small increase in the activity of other ARVI pathogens was registered (up to 16.1% in early May 2023). From mid-September 2023, an increase in the number of positive samples for SARS-CoV-2 was also registered (up to 9.2%).
The frequency of positive findings by PCR results during the analyzed period as a whole for the epidemic season 2022–2023 was as follows: influenza – 9.6% (out of 39 746 examined), ARVI – 10.7% (out of 35 340 examined) and SARS-CoV-2 – 6.2% (out of 18 873 examined). At the same time, their activity differed in various cities of the Russian Federation (Table 1).
Table 1. The results of PCR diagnostics of influenza, SARS-CoV-2 and some acute respiratory infections in the period October 2022 – September 2023 at the National Research Centre of N.F. Gamaleya and in the territories of the Russian Federation cooperating the Center
Таблица 1. Результаты ПЦР-диагностики гриппа, SARS-CoV-2 и некоторых ОРВИ в период октября 2022 г. – сентября 2023 г. в ЦЭЭГ НИЦЭМ им. Н.Ф. Гамалеи и на сотрудничающих с ним территориях РФ
Centers of Hygiene and Epidemiology of cities, regions, republics Центры гигиены и эпидемиологии городов, областей, республик | The number of samples examined for the presence of respiratory pathogens by RT-PCR Число образцов, изученных на наличие респираторных патогенов методом ОТ-ПЦР | |||||||||||
influenza viruses грипп | Acute respiratory infections, seasonal ОРВИ, сезонные | SARS-CoV-2 | ||||||||||
number of samples число образцов | % «+» | number of samples число образцов | HPIV % «+» | HAdV % «+» | HRsV % «+» | HRV % «+» | HCoV % «+» | HBoV % «+» | HMPV % «+» | number of samples число образцов | % «+» | |
Center for Ecology and Epidemiology of Influenza, Moscow ЦЭЭГ, Москва | 1222 | 14,5 | 596 | 2,7 | 2,5 | 4,0 | 12,9 | 3,0 | 1,3 | 2,2 | 1224 | 8,5 |
Veliky Novgorod Великий Новгород | 1728 | 7,7 | 1349 | 1,1 | ||||||||
Lipetsk Липецк | 1214 | 5,5 | 1214 | 2,1 | 3,3 | 2,7 | 1,6 | 0,2 | 0,7 | 1,0 | 1214 | |
Vladimir Владимир | 1481 | 17,3 | 971 | 2,4 | 6,3 | 6,5 | 15,9 | 3,6 | 2,3 | 3,4 | 972 | |
Yaroslavl Ярославль | 3578 | 17,3 | 1683 | 1,0 | 1,3 | 6,6 | 12,7 | 2,3 | 1,1 | 3,6 | 2083 | 5,9 |
Penza Пенза | 1875 | 10,1 | 1136 | 1,8 | 2,4 | 3,5 | 11,6 | 2,6 | 2,0 | 2,6 | 1136 | 5,0 |
Cheboksary Чебоксары | 3448 | 20,1 | 3443 | 0,2 | 0,3 | 0,7 | 0,2 | 0,3 | 6121 | 9,0 | ||
Orenburg Оренбург | 19 194 | 4,0 | 19 320 | 0,3 | 0,2 | 0,9 | 1,6 | 0,6 | 0,3 | 0,6 | ||
Tomsk Томск | 2062 | 13,8 | 2062 | 1,6 | 2,3 | 4,4 | 6,9 | 3,2 | 0,5 | 1,4 | 2062 | 9,5 |
Vladivostok Владивосток | 2241 | 15,2 | 2241 | 0,8 | 1,9 | 1,5 | 4,0 | 0,8 | 0,5 | 0,6 | 2241 | 0,9 |
Birobidzhan Биробиджан | 1702 | 16,6 | 1325 | 14,6 | 4,6 | 8,5 | 16 | 4,8 | 4,2 | 2,6 | 1820 | 6,3 |
Total Всего | 39 745 | 9,6 | 35 340 | 1,2 | 1,0 | 2,0 | 3,8 | 1,1 | 0,6 | 1,0 | 18 873 | 6,2 |
% of the positive samples % из числа положительных | 10,9 | 9,4 | 18,8 | 35,6 | 10,3 | 5,8 | 9,2 | |||||
The highest frequency of positive samples for influenza was observed in Cheboksary, Vladimir and Yaroslavl; seasonal ARVI – in Birobidzhan, SARS-CoV-2 – in Moscow, Cheboksary and Tomsk. The top three in the structure of seasonal ARVI were HRV (35.6%), HRsV (18.8%) and HPIV (10.9%).
During sentinel surveillance, the frequency of positive samples (out of 719 examined) was as follows: influenza – 18.0%, SARS-CoV-2 – 4.2%, and ARVI – 22.3% (including HRsV – 4.7% and HRV – 5.9%).
Russian regions also differed in the proportion of influenza virus types/subtypes (Figure 2). The presented data show that influenza A virus dominated in all territories cooperating with the Influenza Ecology and Epidemiology Center in the 2022–2023 season. In the structure of influenza A virus, A(H1N1)pdm09 was more active, with a 67% share; influenza A(H3N2) virus was detected in isolated cases not in all collaborating territories and its share was 3.4%. Influenza B virus strains were detected in one third of cases (33.0%), with higher activity in certain cities compared to others in the European Russia, such as Cheboksary, Orenburg, Tomsk, Vladivostok, and Birobidzhan.
Fig. 2. The share of influenza viruses during the epidemic season 2022–2023 in different regions of the Russian Federation (according to the Centers of Hygiene and Epidemiology of cities, regions, republics).
Рис. 2. Долевое участие вирусов гриппа в период эпидемического сезона 2022–2023 гг. в разных регионах РФ (по данным центров гигиены и эпидемиологии городов, областей, республик).
During sentinel surveillance, the proportion of influenza A(H1N1)pdm09 virus was 77.0%.
The results of antigenic characterization of 150 strains determined that 118 of them were related to influenza virus A(H1N1)pdm09, 9 were related to A(H3N2), and 23 were related to influenza virus type B (Table 2). The studies were conducted against influenza viruses included in influenza vaccines for the 2022–2023 influenza season for the countries of the Northern Hemisphere [8].
Table 2. Antigenic properties of epidemic strains of influenza A and B viruses isolated in the epidemic season 2022–2023
Таблица 2. Антигенные свойства эпидемических штаммов вирусов гриппа А и В, выделенных в эпидемическом сезоне 2022–2023 гг.
Type/subtype of the influenza virus Тип/подтип вируса гриппа | Influenza virus strains included in influenza vaccines in the 2022–2023 season (relative to homologous titer) Штаммы вирусов гриппа, вошедшие в состав гриппозных вакцин в сезоне 2022–2023 гг. (отношение к гомологичному титру) | The number of strains closely related to the reference serum/the number of studied Число штаммов, близкородственных эталонной сыворотке/число изученных | The total number of strains studied Общее число изученных штаммов |
А(H1N1)pdm09 | A/Victoria/2570/19 А/Виктория/2570/19 (1-1/2 : 1/4) | 116 (98,0%)/ 2 (2,0%) | 119 |
Drift variant Дрейф-вариант (< 1/4) | 0 | ||
А(H3N2) | A/Darwin/9/21 А/Дарвин/9/21 (1-1/2 : 1/4) | 3 (33,3%)/ 3 (33,3%) | 9 |
Drift variant Дрейф-вариант (< 1/4) | 3 (33,3%) | ||
В | Victoria-Like Line Линия Виктория-подобных B/Austria/135941/21 В/Австрия/135941/21(D3) (1-1/2) | 6 (25,0%)/ 15 (65,0%) | 23 |
Victoria-Like Line Линия Виктория-подобных Drift variant Дрейф-вариант (< 1/4) | 2 (9,0%) | ||
Line B/Yamagata-like Линия В/Ямагата-подобных B/Phuket/3073/13 B/Пхукет/3073/13 | 0 | 0 |
A(H1N1)pdm09 strains were isolated in all laboratories that specialized in virus isolation. The first strain was isolated from a patient who became ill on October 24, 2022 (Moscow), the last strain – on January 31, 2023 (Moscow). According to the data of interaction in HAI with a spectrum of diagnostic sera, the close affinity of all 118 strains of influenza A(H1N1)pdm09 virus to the reference virus A/Victoria/2570/19 (vaccine strain in the season 2022–2023) was determined.
A(H3N2) strains were isolated from sporadic cases and not in all collaborating laboratories. The first strain was isolated in Birobidzhan from a patient who fell ill on January 15, 2023; the last strain was isolated in Moscow from a patient who fell ill on September 20, 2023. Three (33.3%) of the 9 studied strains interacted with a serum to the virus A/Darwin/9/21 (vaccine) from 1/2 to all of the homologous titer; 3 (33.3%) isolates interacted up to 1/4 of the homologous titer and 3 (33.3%) – up to 1/8 of the homologous titer.
Out of 23 isolated influenza B virus strains (first in October 2022, Yaroslavl and last on March 17, 2023, Moscow), 6 (25.0%) were closely related to the reference B/Austria/1359417/21 (vaccine) and interacted with serum to this virus up to full homologous titer; 15 strains (65.0%) – interacted up to ¼ of the homologous titer and 2 (9.0%) – up to 1/8 homologous titer.
As part of monitoring the sensitivity of the population of circulating influenza virus strains, we studied the properties of 72 epidemic strains of influenza viruses, including 53 strains of A(H1N1)pdm09, 2 strains of A(H3N2) and 17 strains of influenza B to drugs with antineuraminidase activity. The strains were isolated in different regions of the Russian Federation and all of them were found to have normal sensitivity to oseltamivir and zanamivir; the average drug concentration (IC50) was 0.47 and 0.42 nM for A(H1N1)pdm09 strains, respectively; 0.3 and 0.6 nM for influenza A(H3N2) strains, respectively, and 39.25 and 6.12 nM for influenza B strains, respectively.
Molecular genetic studies were conducted for 52 epidemic strains, including 30 influenza A(H1N1)pdm09 strains, 20 influenza B virus strains, and 2 influenza A(H3N2) virus strains isolated in different regions of the Russian Federation.
The following epidemic strains of influenza A(H1N1)pdm09 viruses were isolated in Moscow (26), Yaroslavl (1), Orenburg (2), and Tomsk (1): EPI_ISL_16738404, EPI_ISL_16738405, EPI_ISL_16738406, EPI_ISL_16738407, EPI_ISL_16738408, EPI_ISL_16738409, EPI_ISL_16738410, EPI_ISL_16738411, EPI_ISL_16738412, EPI_ISL_16738413, EPI_ISL_16738414, EPI_ISL_16738415, EPI_ISL_17395563, EPI_ISL_17395562, EPI_ISL_17395561, EPI_ISL_17395560, EPI_ISL_17395559, EPI_ISL_17395558, EPI_ISL_17395557, EPI_ISL_17395556, EPI_ISL_17395555, EPI_ISL_17395554, EPI_ISL_17831605, EPI_ISL_17831605, EPI_ISL_17831604, EPI_ISL_17831603, EPI_ISL_17831602, EPI_ISL_17831601, EPI_ISL_17831600, EPI_ISL_18054497, EPI_ISL_18054498. All were assigned to clade 6B.1A.5a.2a and carried additional mutations K54Q, A186T, Q189E, E224A, R259K, K308R, I418V in hemagglutinin (HA) relative to vaccine strain A/Wisconsin/588/2019 (culture analog of embryonic vaccine virus A/Victoria/2570/19). Two strains (A/Moscow/46/2022 and A/Moscow/32/2022) also carried the D222N mutation in HA. One strain (A/Moscow/32/2022) carried additional mutations V152I and E172K (Ca1 site in HA). Two strains of influenza A(H1N1)pdm09 virus isolated on MDCK cell lines and passaged on chicken embryos (A/Moscow/32/2022, A/ Moscow/37/2022) carried mutations N162K, D222G.
Influenza A(H3N2) viruses were isolated in Moscow (1) and Birobidzhan (1) and assigned to clade 3C.2a1b.2a.2b (2b), represented by A/Darwin/6/2021 virus with additional mutations in HA (E50K, G53D, F79I, T135A(-GLY), I140K, S156H, S262N): EPI_ISL_17395564 and EPI_ISL_17831606.
The following epidemic strains of influenza B viruses were isolated in Moscow (8), Yaroslavl (1), Orenburg (1), Tomsk (1), Birobidzhan (2) and Vladivostok (5), Veliky Novgorod (2): EPI_ISL_17395553, EPI_ISL_17395552, EPI_ISL_17395551, EPI_ISL_17395550, EPI_ISL_17395549, EPI_ISL_17831599, EPI_ISL_17831598, EPI_ISL_17831597, EPI_ISL_17831596, EPI_ISL_17831595, EPI_ISL_18054497, EPI_ISL_18054498, EPI_ISL_18054490, EPI_ISL_18054491, EPI_ISL_18054492, EPI_ISL_18054493, EPI_ISL_18054494, EPI_ISL_18054495, EPI_ISL_18054495, EPI_ISL_18054499. All of them were assigned to the genetic lineage B/Victoria-like (clade V1A.3a.2) represented by B/Austria/1359417/2021. Phylogenetic analysis revealed genetic heterogeneity of influenza B viruses: certain strains carried substitutions in HA relative to vaccine B/Austria/1359417/2021; 12 strains carried substitutions E128K, A154E, S205P, of which 3 strains (B/V.Novgorod/6/2023, B/V.Novgorod/7/2023, B/Moscow/5/2023) carried additional mutations V87I and T121N. Other 5 strains carried the D194E substitution, including 1 strain from Birobidzhan (B/Birobidzhan/2/2023) carrying additional substitutions R80G, E181K.
No genetic markers of resistance to antiviral drugs of the neuraminidase inhibitor group were detected among the influenza virus strains tested.
As part of the sentinel surveillance of patients with acute respiratory infections, data on the effectiveness of influenza vaccine prophylaxis among hospitalized patients were obtained. 15 of the hospitalized patients (719 patients) had a history of influenza vaccination; however, only three had confirmed influenza infection (2 with A(H1N1)pdm09 and 1 with influenza B). Vaccination efficacy, as calculated using the test-negative approach (WHO) was 75.0%. First of all, this confirms the fact that vaccination can prevent the development of more severe forms of influenza infection which would require hospitalization of patients. It was not possible to evaluate the effectiveness of vaccine prophylaxis by other indicators, such as severity of the disease course, frequency of complications, and mortality in this study due to the small sample of vaccinated patients with laboratory-confirmed influenza infection.
Discussion
In contrast to the previous epidemic season in Russia, similar to the countries of the Northern Hemisphere, a relatively early increase in influenza virus activity was observed during the 2022–2023 epidemic season and, despite an increase in the volume of tested samples, the frequency of positive samples for influenza did not reach the indicators of the 2018–2019 pre-pandemic SARS-CoV-2 season. [5, 10, 12]. However, compared to the previous epidemic seasons (2020–2022), a higher activity of influenza viruses was noted both in Russia and other countries with a characteristic cyclical change of the dominant influenza A virus subtype.
Another peculiarity of the period under consideration is that the activity of the new coronavirus was significantly lower in all countries compared to the previous season, with the exception of countries in the Pacific region, where a sharp increase in SARS-CoV-2 activity was recorded in December 2022 and January 2023 [1].
According to WHO, from October 1, 2022 to June 30, 2023, about 9 million samples of clinical materials were tested in all countries of the world, of which 11.2% were positive for influenza viruses [13]. Influenza virus activity had a two-wave pattern due to etiologically different influenza A subtypes: the first wave was observed in the period of November 2022 to January 2023 (with peaks of up to 21.0% of positive samples during weeks 48–52 of 2022) and was associated with greater influenza A(H3N2) virus activity; the second wave was recorded during March–April 2023 (with peaks of up to 11.0% of positive samples during weeks 9–12) and was associated with greater influenza A(H1N1)pdm09 and influenza B virus activity. By week 26 of 2023, the incidence of influenza virus-positive samples among those tested was 2.6%, except in a few countries where rates were higher – Norway (up to 25.0%), Iraq (up to 25.0%), Senegal (up to 40.0%), etc.; SARS-CoV-2 was 9.2%, except in a few countries such as Norway (up to 44.0%), Ukraine (up to 57.0%) and Chile (up to 49.0%), among others.
Over the entire period analyzed, the proportion of influenza viruses was as follows: influenza type A, 82.8%, and influenza type B, 17.2%; 51.0% of influenza A subtyped viruses were A(H1N1)pdm09 and 49.0% were A(H3N2); all of the influenza B virus-positive samples studied were attributed to the B/Victoria-like lineage. Differences in the proportion of influenza viruses in WHO countries and regions were observed, as in previous seasons.
In WHO European region countries, virus activity varied, including the predominant influenza virus [11]. In countries such as Switzerland, Turkey, Luxembourg, Slovakia, Spain and Sweden, influenza A(H3N2) virus dominated early in the season, followed by influenza B virus in February–March 2023. In Norway, Moldova, Romania and the United Kingdom, as in Russia, influenza B was replaced by influenza A(H1N1)pdm09; the etiology of the epidemics in Malta and Portugal was influenza A(H3N2) and in Serbia by A(H1N1)pdm09.
Influenza A(H3N2) dominated in most countries in the WHO Americas Region, with the exceptions of Argentina (influenza B), Venezuela (A(H1N1)pdm09) and Canada (A(H1N1)pdm09+A(H3N2)) [14, 15].
The activity of all 3 influenza viruses was monitored in countries in the WHO South-East Asia Region; influenza A(H3N2) virus predominated between October 2022 and mid-March 2023, followed by co-circulation of influenza A(H3N2) and B viruses. In Bangladesh, influenza B virus was dominant, while in India, co-circulation of all three viruses was recorded in the second half of the season [15].
Co-circulation of A(H1N1)pdm09 (predominant) and A(H3N2) has been reported in WHO Western Pacific Region countries [15].
In WHO African Region countries, influenza A(H1N1)pdm09 virus activity has been high since October 2022, influenza A virus co-circulation in April, and influenza A(H3N2) virus activity in May; influenza B virus has been diagnosed in sporadic cases [15].
In WHO Eastern Mediterranean Region countries during the period under review, influenza A (predominantly A(H3N2)) was diagnosed as the predominant influenza A virus, with high activity of influenza A(H1N1)pdm09 in several countries, such as Afghanistan, Jordan and Pakistan; co-circulation of A(H1N1)pdm09 and B was recorded in Tunisia and Iraq; influenza B in Saudi Arabia [15].
A study of the antigenic and genetic properties of the circulating strain population (more than 10,000 in the United States and European countries) revealed, for the most part, their complete correspondence to the strains included in influenza vaccines in the 2022–2023 season for the countries of the Northern Hemisphere (with the exception of influenza virus A(H1N1)pdm09) [11, 14]. The identified mutations did not change the antigenic properties of epidemic strains in the population as a whole, which also did not affect vaccine efficacy.
The work of our colleagues at the A.A. Smorodintsev Research Institute of Influenza of the Russian Ministry of Health presents data on the evaluation of the effectiveness of influenza vaccine prophylaxis in the context of the data obtained by studying the antigenic and genetic properties of influenza viruses circulating in Russia in the 2022–2023 season [10]. Samples were collected from patients as part of sentinel (SARI – 1,631 patients) and traditional surveillance (FLI and ARI – 1,178 patients). The authors noted low vaccination coverage among patients in both groups (0.7% for SARI and 6.6% for FLI and ARI), with vaccine prophylaxis efficacy among vaccinated patients of 92.7% and 54.7%, respectively (mean, 80.0%). These data are consistent with the results obtained by the authors of this work on the effectiveness of vaccine prophylaxis with regard to the rate of hospitalization of patients with influenza infection, although they were obtained on a smaller sample of patients.
By February 2023, the population of epidemic strains of influenza A(H1N1)pdm09 virus was represented by two phylogenetic clades: 5a.1 and 5a.2, with a higher frequency of representation of the latter clade. Since 2022, further processes in the acquisition of new mutations in the hemagglutinin of representatives of clade 5a.2 have been traced with the formation of subclades: 5a.2a (+K54Q, A186T, Q189E, E224A, R259K and K308R in the Sb site); 5a.2a.1, represented by A/Wisconsin/67/22 (P137S, K142R, D260E, T277A and for the majority of them – T216A). When examining the levels of specific antibodies acquired by volunteers after vaccination with the recommended formulation for the 2022–2023 epidemic season in February 2023, significant reductions in geometric mean titers (GMTs) were recorded in most samples to circulating influenza A(H1N1)pdm09 virus strains of subclades 5a.2a and 5a.2a.1 in most samples. In this regard, WHO experts revised the composition of influenza vaccines for the countries of the Northern Hemisphere in 2023–2024 with the replacement of only one of the components – A(H1N1)pdm09 (A/Victoria/2579/2019 to A/Victoria/4897/2022). February 25, 2023. WHO published recommendations on influenza vaccine formulations for countries in the Northern Hemisphere for the 2023–2024 season [16]. Trivalent chicken embryo-based vaccines will contain a virus similar to A/Victoria/4897/2022 (H1N1)pdm09; a virus similar to A/Darwin/9/2021 (H3N2) and a virus similar to B/Austria/1359417/2021 (B/Victoria-like lineage). Influenza virus B/Phuket/3073/2013 (B/Yamagata-like lineage) is recommended in quadrivalent chicken embryo-based vaccines.
By September 2023, it was clear that the population of influenza A(H3N2) virus strains, clade 3C.2a1b.2a2, had also changed from the last of the reference variants, A/Darwin/16/2021 (2a), and was represented by genetic groups 2a (with variants 2a–2d) and the dominant 2a.3a.1 (A/Thailand/8/2022). Differences in the dominance of a particular variant of influenza A(H3N2) virus were noted for the countries of the world: variants 2a1b circulated mainly in Europe and North America; 2a.3a.1 – in Africa, Asia, North America and Oceania; 2b – in every country of the world. When examining the levels of specific antibodies acquired by volunteers after vaccination with the recommended formulation for the 2022–2023 (Northern Hemisphere) and 2023 (Southern Hemisphere) epidemic seasons, significant reductions in the geometric mean titers (GMTs) were recorded in most samples against circulating strains of influenza A(H3N2)pdm09 subclade 2a.3a.1. Therefore, WHO experts revised the composition of influenza vaccines for countries of the Southern Hemisphere in 2024 with the replacement of the influenza A(H3N2)pdm09 virus component (A/Darwin/16/2021 to A/Thailand/8/2022) [17]. These changes in the properties of influenza A(H3N2) virus may cause lower efficacy of influenza vaccines in the countries of the Northern Hemisphere in the current season (2023–2024) and especially in those countries where its activity will be dominant (e.g., Russia).
We analyzed six studies evaluating influenza vaccine effectiveness (IVE) in the 2022–2023 season based on data from 16 European countries during the influenza monitoring period among outpatients and hospitalized patients, including those in intensive care units [18]. As mentioned above, the proportion of influenza viruses involved during the epidemic upsurge varied between countries in the European region, in particular, the frequency of positive samples for influenza A ranged from 17.0 to 95.0%. Preliminary data showed that the efficacy of vaccine prophylaxis against influenza this virus type ranged from 27.0 to 44.0%, influenza B virus more than 50.0%, with a higher rate in children (50.0–90.0%) compared with adults (12.0–49.0%). For influenza A(H1N1)pdm09 virus, the IVE was 28.0 to 46.0%, while the same differences were noted in children (49.0–77.0%) and adults (21.0–56.0%); for influenza A(H3N2) virus, the rates ranged from 2.0 to 44.0% and in the aforementioned age groups, they were 62.0–70.0 and 36.0–42.0% respectively; for influenza B, rates were presented in the paper only for children under 18 years of age (88.0–90.0%) and 2–6 years of age (87.0–95.0%). The authors note the close affinity of the epidemic strains in antigenic properties to the vaccine virus (A/Darwin/6/2021) and their belonging to the 3C.2a1b.2a.2 clade, with a rather high frequency of genetic diversity in the general population, which may have influenced the differences obtained by other investigators. For example, Canadian researchers reported data somewhat different from those mentioned above for countries in the European region and pointed out the following: vaccine efficacy against influenza A(H3N2) virus among all patients was 58.0–59.0% (higher) and children 47.0% (lower) [19]. The authors suggest the possible influence of a mutation (T135K) in hemagglutinin with loss of the glycosylation site, which was more frequent in the population of epidemic strains isolated from patients younger than 25 years of age.
In WHO European region countries, influenza B viruses had relatively low activity during epidemic rises from 2019–2020, with higher vaccine efficacy [20]. The same data for the B-virus component of vaccines have been reported in other countries around the world (Canada and USA) [21, 22]. The results of molecular genetic studies of the population of influenza B virus strains in the period 2020–2023 determined that they belong to the B/Victoria-like lineage, clade V1A.3a.2 [11, 14, 19]. Epidemic strains from recent years contained substitutions in positions leading to phenotypic reversion to viruses with similar antigenic properties circulating more than 50 years ago [23]. Differences in the efficacy of influenza vaccines in different age groups may also be explained by potential imprinting effects (human immunologic memory from previously encountered influenza viruses).
As mentioned above, current quadrivalent influenza vaccines contain two influenza viruses (A(H1N1)pdm09 and A(H3N2)) and two influenza B viruses of different evolutionary lineages (B/Victoria-like and B/Yamagata-like), which separated in the 1970s and have been co-circulating since then [24, 25]. Since March 2020, each country that monitors the circulation of influenza viruses has begun to note the absence of B/Yamagata-like influenza viruses in active circulation. Therefore, WHO recommends that surveillance of this influenza B virus variant should not be suspended and that molecular genetic testing (sequencing) should be expanded for samples not typed by public health test systems. If the extinction of the influenza B virus lineage B/Yamagata-like is officially recognized, the relevance of quadrivalent vaccines could be reconsidered, for example, by switching to trivalent preparations or adding certain components of other influenza virus types/subtypes [24].
Studies of more than 13,000 samples of epidemic strains of influenza viruses in the United States and European countries have determined their favorable sensitivity profile to drugs with antineuraminidase activity (oseltamivir and zanamivir) and an inhibitor of the enzyme in the polymerase acidic subunit of the viral RNA polymerase complex (baloxavir): reduced sensitivity to the drugs was registered in single cases, which was no more than 1.0% [10, 13]. It should be noted that only in relation to influenza A and B viruses, drugs with a direct mechanism of action have been developed and put to use, the effectiveness of which has been proven by many researchers. Due to this, strategic stocks have been made in case of a new influenza pandemic [26, 27].
Furthermore, an integrated approach to forecasting the development of the epidemic process of ARVI against the background of the emergence of new pathogens (disease X) with the involvement of such possible methods as observation, descriptive (descriptive-evaluation models/methods), analytical and experimental methods, mathematical modeling is obvious today.
Conclusion
The epidemic season of 2022–2023 had its own peculiarities and especially against the background of relatively low activity of SARS-CoV-2 and its new variants, was characterized by an earlier onset, the highest activity of influenza A virus, and the countries of the world differed by the dominant subtype (A(H1N)pdm09 and A(H3N2)), as well as the share of influenza B virus of the B/Victoria-like lineage; influenza virus of the B/Yamagata-like lineage has not been active since March 2020. Depending on the activity of the influenza virus type/subtype at certain periods of the season, differences were observed in the incidence, age group involvement, mortality and efficacy of influenza vaccines. In terms of antigenic and molecular genetic properties, the population of epidemic strains of influenza viruses was similar to the viruses included in the influenza vaccines recommended by WHO experts for the 2022–2023 season, higher efficacy was observed for the A(H1N1)pdm09 component (especially in countries where it was dominant), and lower efficacy for the A(H3N2) component, which was the reason for its replacement in the vaccines for the countries of the Southern Hemisphere for the 2024 season; a favorable sensitivity profile to drugs with antineuraminidase activity, as well as to an inhibitor of the enzyme that synthesizes the influenza virus matrix RNA was maintained. All of the above points to the relevance of the research and the data obtained as part of the ongoing surveillance of influenza virus circulation.
About the authors
Elena I. Burtseva
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Author for correspondence.
Email: elena-burtseva@yandex.ru
ORCID iD: 0000-0003-2518-6801
Dr. Sci. (Medicine), Head of influenza etiology and epidemiology laboratory National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowLudmila V. Kolobukhina
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: lkolobuchina@yandex.ru
ORCID iD: 0000-0001-5775-3343
Dr. Sci. (Medicine), prof., Head of Department, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowAnna D. Panova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: ainushgnomello@gmail.com
ORCID iD: 0000-0002-9322-6273
junior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowEvgeniya A. Mukasheva
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: mukasheva_evgeniya@mail.ru
ORCID iD: 0000-0002-5688-5309
researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowKirill G. Krasnoslobodtsev
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: kkg_87@mail.ru
ORCID iD: 0000-0003-1745-9128
researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowElena S. Kirillova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: esshevchenko@yandex.ru
ORCID iD: 0000-0001-7977-7530
PhD (Medicine), leading researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowNatalia V. Breslav
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: n.belyakova1983@gmail.com
ORCID iD: 0000-0002-6946-5119
PhD (Biology), senior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowSvetlana V. Trushakova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: s.trushakova@gmail.com
ORCID iD: 0000-0002-9610-3041
PhD (Biology), senior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowIrina A. Komarova
Pacific State Medical University of the Ministry of Health of the Russian Federation
Email: mikhaira@yandex.ru
ORCID iD: 0000-0003-0483-7433
assistent, Department of Infectious Diseases, Pacific State Medical University of the Ministry of Health of the Russian Federation
Russian Federation, 690002, Primorsky Krai, VladivostokElena L. Feodoritova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: flulab@mail.ru
ORCID iD: 0000-0002-1472-1357
researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowLiliya N. Merkulova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: merkulova0320@yandex.ru
ORCID iD: 0000-0002-7260-0879
PhD (Medicine), leading researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowIrina N. Khlopova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: khlopova.ira@yandex.ru
ORCID iD: 0000-0002-7419-590X
PhD (Medicine), leading researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowIrina S. Kruzhkova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: irina-kru@yandex.ru
ORCID iD: 0000-0002-1983-481X
junior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowAnna V. Ignatieva
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: valgella@yandex.ru
ORCID iD: 0000-0001-6206-2299
PhD (Biology), senior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowAnastasia S. Krepkaia
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: nastya18-96@mail.ru
ORCID iD: 0000-0002-7272-4011
junior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowAndrey B. Komissarov
Research institute of influenza named after A.A. Smorodintsev of Ministry of Health
Email: andrey.komissarov@influenza.spb.ru
ORCID iD: 0000-0003-1733-1255
Head of molecular virology laboratory, Research institute of influenza named after A.A. Smorodintsev of Ministry of Health
Russian Federation, 197022, St. PetersburgAndrei A. Pochtovyi
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: a.pochtovyy@gamaleya.org
ORCID iD: 0000-0003-1107-9351
PhD (Biology), senior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowDaria D. Kustova
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: kustovad70@gmail.com
ORCID iD: 0000-0002-8382-275X
junior researcher, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowVladimir A. Gushchin
National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Email: wowaniada@yandex.ru
ORCID iD: 0000-0002-9397-3762
Dr. Sci. (Biology), Head of laboratory, National Research Centre of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya of Ministry of Health
Russian Federation, 123098, MoscowIgor N. Tyurin
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: tyurin.dti@yandex.ru
ORCID iD: 0000-0002-5696-1586
PhD (Medicine), chief physician, Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Russian Federation, 125367, MoscowAlexey A. Samkov
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: a.a.samkov@yandex.ru
ORCID iD: 0000-0002-0365-3096
deputy chief physician, Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Russian Federation, 125367, MoscowNatalya А. Antipyat
Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Email: natadoc70@bk.ru
ORCID iD: 0000-0001-8578-2838
deputy chief physician, Clinical Hospital for Infectious Diseases No. 1, Department of Health of Moscow
Russian Federation, 125367, MoscowReferences
- Coronavirus infection (COVID-19). Available at: https://who.int/emergencies/diseases/novel-coronavirus-2019
- Sanz I., Perez D., Rojo S., Domínguez-Gil M., de Lejarazu R.O., Eiros J.M. Coinfections of influenza and other respiratory viruses are associated to children. An. Pediatr. (Engl. Ed). 2022; 96(4): 334–41. https://doi.org/10.1016/j.anpede.2021.03.002
- Sominina A.A., Danilenko D.M., Stolyarov K.A., Karpova L.S., Bakaev M.I., Levanyuk T.P., et al. Interference of SARS-CoV-2 with other respiratory viral infections agents during pandemic. Epidemiologiya i vaktsinoprofilaktika. 2021; 20(4): 28–39. https://doi.org/10.31631/2073-3046-2021-20-4-28-39 https://elibrary.ru/cdrnsj (in Russian)
- Akimkin V.G., Popova A.Yu., Ploskireva A.A., Ugleva S.V., Semenenko T.A., Pshenichnaya N.Yu., et al. COVID-19: the evolution of the pandemic in Russia. Report I: manifestations of the covid-19 epidemic process. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2022; 99(3): 269–86. https://doi.org/10.36233/0372-9311-276 https://elibrary.ru/zxgtfd (in Russian)
- Burtseva E.I., Kolobukhina L.V., Voronina O.L., Ignat’eva A.V., Mukasheva E.A., Panova A.D., et al. Features of the circulation of ARVI pathogens during of emergence and widespread of SARS-CoV-2 in the 2018–2021. Epidemiologiya i vaktsinoprofilaktika. 2022; 21(4): 16–26. https://doi.org/10.31631/2073-3046-2022-21-4-16-26 https://elibrary.ru/rnyfoi (in Russian)
- Burtseva E.I., Panova A.D., Kolobukhina L.V., Ignat’eva A.V., Kirillova E.S., Breslav N.V., et al. Epidemic season 2021-2022: frequency of co-infection by respiratory viral pathogens. Epidemiologiya i infektsionnye bolezni. 2023; 28(2): 67–77. https://doi.org/10.17816/EID321873 https://elibrary.ru/mdoeta (in Russian)
- WHO. Evaluation of influenza vaccine effectiveness. A guide to the design and interpretation of observational studies; 2017. Available at: https://who.int/publications/i/item/9789241512121
- Zhou B., Lin X., Wang W., Halpin R.A., Bera J., Stockwell T.B., et al. Universal influenza B virus genomic amplification facilitates sequencing, diagnostics, and reverse genetics. J. Clin. Microbiol. 2014; 52(5): 1330–7. https://doi.org/10.1128/JCM.03265-13
- Zhou B., Donnelly M.E., Scholes D.T., St. George K., Hatta M., Kawaoka Y., et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses. J. Virol. 2009; 83(19): 10309–13. https://doi.org/10.1128/JVI.01109-09
- Sominina A., Danilenko D., Komissarov A.B., Pisareva M., Fadeev A., Konovalova N., et al. Assessing the intense influenza A(H1N1)pdm09 epidemic and vaccine effectiveness in the post-COVID season in the Russian Federation. Viruses. 2023; 15(8): 1780. https://doi.org/10.3390/v15081780
- WHO. Recommended composition of influenza virus vaccines for use in the 2022–2023 northern hemisphere influenza season; 2022. Available at: https://who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022-2023-northern-hemisphere-influenza-season
- ECDC. Seasonal influenza – Annual epidemiological report for 2022/2023. Available at: https://ecdc.europa.eu/en/publications-data/seasonal-influenza-annual-epidemiological-report-20222023
- WHO. Global influenza programme. Influenza updates. Available at: https://who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-updates
- CDC. Weekly U.S. Influenza Surveillance Report; 2024. Available at: http://cdc.gov/flu/weekly/index.htm/
- WHO. Global influenza programme. Influenza surveillance outputs. Available at: https://who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-surveillance-outputs
- WHO. Recommendations announced for influenza vaccine composition for the 2023-2024 northern hemisphere influenza season. Available at: https://who.int/news/item/24-02-2023-recommendations-announced-for-influenza-vaccine-composition-for-the-2023-2024-southern-hemisphere-influenza-season
- WHO. Recommendations announced for influenza vaccine composition for the 2023-2024 southern hemisphere influenza season. Available at: https://who.int/news/item/24-02-2023-recommendations-announced-for-influenza-vaccine-composition-for-the-2023-2024-northern-hemisphere-influenza-season
- Kissling E., Maurel M., Emborg H.D., Whitaker H., McMenamin J., Howard J., et al. Interim 2022/23 influenza vaccine effectiveness: six European studies, October 2022 to January 2023. Euro Surveill. 2023; 28(21): 2300116. https://doi.org/10.2807/1560-7917.ES.2023.28.21.2300116
- Skowronski D.M., Chuang E.S., Sabaiduc S., Kaweski S.E., Kim S., Dickinson J.A., et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Euro Surveill. 2023; 28(5): 2300043. https://doi.org/10.2807/1560-7917.ES.2023.28.5.2300043
- Rose A., Kissling E., Emborg H.D., Larrauri A., McMenamin J., Pozo F., et al. Interim 2019/20 influenza vaccine effectiveness: six European studies, September 2019 to January 2020. Euro Surveill. 2020; 25(10): 2000153. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000153
- Skowronski D.M., Zou M., Sabaiduc S., Murti M., Olsha R., Dickinson J.A., et al. Interim estimates of 2019/20 vaccine effectiveness during early-season co-circulation of influenza A and B viruses, Canada, February 2020. Euro Surveill. 2020; 25(7): 2000103. https://doi.org/10.2807/1560-7917.ES.2020.25.7.2000103
- Dawood F.S., Chung J.R., Kim S.S., Zimmerman R.K., Nowalk M.P., Jackson M.L., et al. Interim Estimates of 2019-20 Seasonal Influenza Vaccine Effectiveness – United States, February 2020. MMWR Morb. Mortal. Wkly Rep. 2020; 69(7): 177–82. https://doi.org/10.15585/mmwr.mm6907a1
- Rosu M.E., Lexmond P., Bestebroer T.M., Hauser B.M., Smith D.J., Herfst S., et al. Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc. Natl Acad. Sci. USA. 2022; 119(42): e2211616119. https://doi.org/10.1073/pnas.2211616119
- Paget J., Caini S., Del Riccio M., van Waarden W., Meijer A. Has influenza B/Yamagata become extinct and what implications might this have for quadrivalent influenza vaccines? Euro Surveill. 2022; 27(39): 2200753. https://doi.org/10.2807/1560-7917.ES.2022.27.39.2200753
- Virk R.K., Jayakumar J., Mendenhall I.H., Moorthy M., Lam P., Linster M., et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl Acad. Sci. USA. 2020; 117(1): 619–28. https://doi.org/10.1073/pnas.1916585116
- CDC. Influenza Antiviral Medications: Summary for clinicians. Available at: https://cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Uyeki T.M., Bernstein H.H., Bradley J.S., Englund J.A., File T.M., Fry A.M., et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019; 68(6): 895–902. https://doi.org/10.1093/cid/ciy874
Supplementary files