Пререгистрированный мета-метаанализ глобального распространения гепатотропных вирусов
- Авторы: Adeiza S.1,2, Islam M.3,4, Mungadi H.5, Shuaibu A.5, Sah R.6,7
-
Учреждения:
- Университет Ахмаду Белло
- Усману Дафодийо Университет
- Медицинский колледж президента Абдула Хамида
- Университет науки и технологий Ноакхали
- Усману Данфодио Университет
- Институт медицины
- Медицинский колледж, больница и научно-исследовательский центр Д.Д. Патиля, Университет Д.Д. Патиля
- Выпуск: Том 69, № 5 (2024)
- Страницы: 429-440
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://virusjour.crie.ru/jour/article/view/16639
- DOI: https://doi.org/10.36233/0507-4088-234
- EDN: https://elibrary.ru/txidjt
- ID: 16639
Цитировать
Полный текст
Аннотация
Введение. Гепатотропные вирусы (ВГА, ВГВ, ВГС, ВГD и ВГЕ) значительно влияют на мировое здравоохранение при их различной распространенности в разных регионах мира.
Цель исследования. Настоящее исследование направлено на систематическую консолидацию данных из разных метаанализов для оценки распределения и распространенности гепатотропных вирусов.
Материалы и методы. Следуя рекомендациям PRISMA, в исследовании использовали модель смешанных эффектов для интеграции данных. Оценку качества проводили с использованием инструментов QUOROM и AMSTAR, с оценкой гетерогенности по статистикам I2 Хиггинса, Q-статистике и значениям тау квадрат (τ2).
Результаты. В исследование проанализированы 86 метаанализов из 56 исследований (2017–2022 гг.) с минимальным перекрытием. Распространенность по регионам была следующей: MENA – 29,2%, Афганистан – 9,14%, Африка – 8,10%. Распространенность подгрупп вирусов: ВГА – 82,5%, ВГВ – 8,6%, ВГС – 15,1%, ВГD – 8,9%, ВГЕ – 13,9%, двойная коинфекция ВГВ/ВГС – 2,2%. Распространенность по группам риска: общее население – 8,3%, медицинские работники – 4,0%. Распространенность ВГВ/ВГС по континентам: Африка – 9,2%, Китай – 6,9% и др. Распространенность ВГС среди групп риска: медицинские работники – 5,58%, пациенты на гемодиализе – 34,8%. Региональные показатели распространенности ВГС: Африка – 7,42%, Ближний Восток – 25,30%.
Заключение. Различия паттернов распространенности гепатотропных вирусов в разных регионах мира обусловлены вляинием многих факторов. Для стран MENA более высокие показатели распространенности связаны с проблемами в здравоохранении, в то время как для Африки наибольшей проблемой являются ограниченные ресурсы. Необходимы индивидуализированные стратегии общественного здравоохранения, включая вакцинацию и информационно-просветительские кампании, для смягчения бремени и улучшения мирового здравоохранения. Полученные консолидированные данные о распространенности гепатотропных вирусов послужат ценным источником информации для принятия обоснованных решений.
Ключевые слова
Полный текст
Introduction
Hepatotropic viruses, including Hepatitis A virus (HAV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Hepatitis D virus (HDV), and Hepatitis E virus (HEV), present a formidable global health challenge [1]. These viruses have different effects on the liver, ranging from mild acute hepatitis to potentially fatal conditions such as cirrhosis and hepatocellular carcinoma [1, 2]. A comprehensive understanding of these viral agents, specifically their prevalence, transmission dynamics, preventive strategies, treatment outcomes, economic implications and associated challenges, is imperative to effectively address their intricate impact on global health as they cause infection in both humans and animals. Aside from HEV, the rest of the hepatitis viruses are only able to infect humans and apes. HAV can also infect a wide range of non-human primates. Athough other hepadnaviruses and hepaciviruses are present in different animal species, evidence supporting the presence of HBV or HCV in animals have not been reported.
Chronic HBV and HCV infections stand out among hepatotropic viruses due to their prolonged global health impact [2]. These infections not only contribute substantially to the worldwide disease burden but also incur significant economic costs through healthcare expenses and reduced productivity [3]. HBV related viruses have been found in primates, rodents, bats and birds. Infection with HCV has been reported in humans and chimpanzees only, while HEV genotypes 1 and 2 cause infections only in humans, genotypes 3 and 4 infect animals and can be transmitted between humans and other animal species. HEV causing liver diseases can have zoonotic origin. Transmission is associated with animal contact and ingestion of raw or uncooked meat, especially the liver. Success in navigating the complexities of chronic HBV and HCV infections requires effective preventive measures, early diagnosis and accessible treatment options to mitigate the individual, societal and economic burdens [3].
Global efforts have been undertaken to combat hepatotropic viral infections, with successful vaccines against HAV and HBV leading to reduced infection rates, particularly among high-risk groups and infants. Moreover, the advent of direct-acting antivirals (DAAs) has revolutionized HCV treatment by achieving unprecedented cure rates [4]. Despite these advancements, challenges such as unequal vaccine coverage, limited diagnostic and treatment access, as well as the emergence of drug resistance persist as obstacles in the pursuit of controlling these infections [2].
Multiple meta-analyses are conducted annually to explore diverse aspects of hepatotropic viral infections, each focusing on specific characteristics of the infections [5–9]. However, these analyses often lack a comprehensive perspective and may inadvertently overlook critical nuances.
While remarkable therapeutic progress has been made, the economic viability of interventions remains a pressing concern. Chronic HCV infections are particularly prevalent, affecting more than 56.8 million individuals globally in 2020 [10]. However, the availability of oral DAAs, which have transformed HCV treatment outcomes, comes at a substantial cost [11]. A study highlighted the exorbitant expenses, with a single pill of a hepatitis C drug priced at $1,000 and a 12-week treatment regimen totaling $84,000 [11]. Furthermore, another DAA was identified with a monthly cost of $23,600, potentially extending treatment duration to six months or a year. Cirrhosis also incurs significant expenses, with approximately 66% of liver-related expenditures in the United States (totaling $32.5 billion with a 95% confidence interval ranging from $27.0 billion to $40.4 billion) linked to hospital stays or urgent care visits [2].
Amid the complexities of diagnosis and treatment affordability, a universal screening strategy emerges as a viable solution to enhance screening and diagnosis rates for hepatotropic viral infections. This strategy could lead to early detection, allowing for timely intervention and mitigating the long-term health and economic impacts associated with chronic infections. Furthermore, universal screening could play a pivotal role in global public health initiatives aimed at achieving the ambitious World Health Organization (WHO) goal of eliminating viral hepatitis as a public health threat by 2030.
The meta-meta-analysis approach, which synthesizes data from diverse meta-analyses, offers a more comprehensive and interconnected perspective [12]. By amalgamating insights from various meta-analyses on HAV, HBV, HCV, HDV and HEV, this approach has the potential to uncover overarching trends, identify disparities and address knowledge gaps more effectively. In light of these considerations, our meta-meta-analysis seeks to provide a comprehensive and multifaceted perspective of hepatotropic viral infections, informing evidence-based interventions and underscoring the importance of universal screening strategies.
Materials and methods
This meta-meta-analysis was designed to estimate the accumulated burden of hepatotropic viruses from various meta-analyses, providing a more comprehensive and interconnected perspective. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses or PRISMA [13] guidelines for its literature review, employing a comprehensive search across Scopus, PubMed and Google Scholar databases, with specific search strings provided in supplementary files. The study was pre registered at the Open Science Framework (osf.io/hzy3n). The focus of this review encompassed systematic reviews and meta-analyses investigating the collective prevalence of hepatitis viruses (HAV, HBV, HCV, HDV and HEV). Eligible meta-analytic research was required to follow PRISMA guidelines, employ the REM (random effects model) method for data aggregation, and disclose viral detection techniques (ELISA, ICT or PCR). Setting type and publication year were not limiting factors, while excluded studies lacked proper data pooling through REM analysis, well-defined search algorithms, or clear selection criteria. Studies without information about the population at risk were also excluded.
Data extracted from included meta-analyses covered study names, publication years, sample sizes, effect size data, populations at risk, number of searched databases and review year ranges. Aggregation was performed based on event counts and corresponding sample sizes. In cases where sample sizes were absent, evidence was derived from effect size data, and missing event numbers were synthesized using point estimates and sample size details. The mixed effects model was applied to integrate these aggregation techniques, with the analysis conducted using Comprehensive Meta-Analysis (CMA Version 4).
The quality and robustness of included meta-analyses were assessed using the Quality of Reporting of Meta-analyses (QUOROM) [14] checklist and the Assessment of Multiple Systematic Reviews (AMSTAR) [15, 16]. The heterogeneity assessment utilized the Higgins I² statistic, where values below 25% indicated low heterogeneity, and values above 75% indicated high heterogeneity [17, 18]. The Q-statistic measures variability in meta-analysis. Low values imply chance, high values show substantial heterogeneity [19]. Tau squared (τ²) measures between-study variance and true heterogeneity. High values signify greater variation beyond chance [20].
Results
This study incorporated 86 meta-analyses from 55 studies [5‒9, 21–66], covering an average sample size of 1,070,005 samples from 553 distinct studies conducted between 1964 and 2019 (Fig. 1 and Table S1). The included meta-analyses, published between 2017 and 2022, exhibited a low degree of overlap (0.0009875847) in their primary studies. The prevalence of hepatotropic viruses ranged from 0.70% to 92.0%. The assessed meta-analyses varied in AMSTAR and QUOROM scores, spanning from 7 to 10 and 9 to 14, respectively.
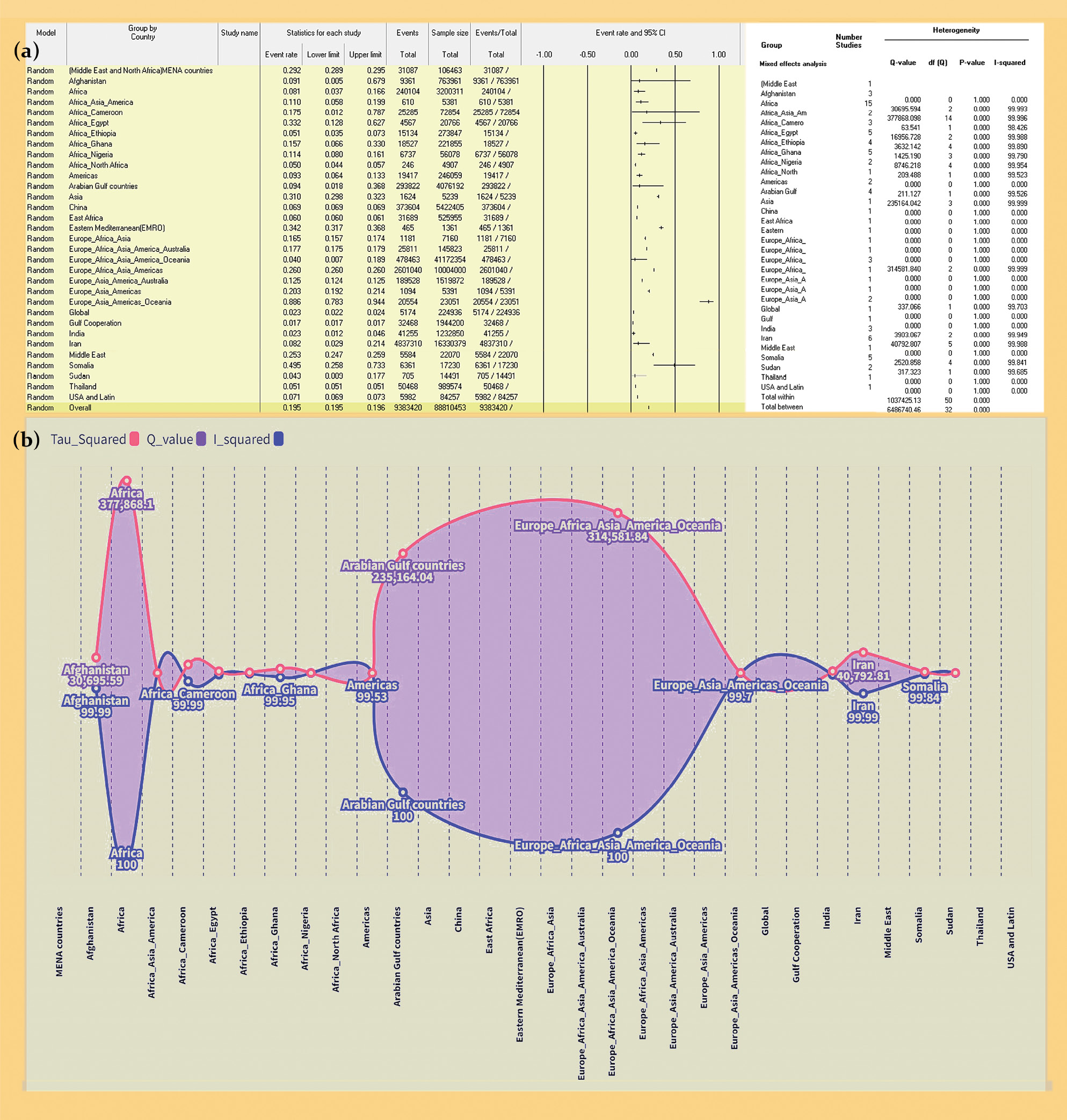
Fig. 1. PRISMA flow chart of included meta-analysis.
Рис. 1. Блок-схема PRISMA включенных метаанализов.
Distribution and prevalence of hepatotropic viruses across diverse geographical regions: The meta-meta-analysis included 55 studies examining the burden of hepatotropic viruses across regions (Fig. 2 a). The accumulated prevalence rates for different regions were as follows: MENA countries (29.2%, 95% CI: 28.93 to 29.47), Afghanistan (9.14%, 95% CI: 0.48 to 67.3), Africa (8.10%, 95% CI: 3.70 to 16.7), Africa-Asia-America combined (11.03%, 95% CI: 5.84 to 19.3), Cameroon (17.55%, 95% CI: 1.21 to 78.3), Egypt (33.20%, 95% CI: 12.81 to 62.3), Ethiopia (5.07%, 95% CI: 3.50 to 29.3), Ghana (15.68%, 95% CI: 6.57 to 32.3), Nigeria (11.39%, 95% CI: 7.96 to 16.3), North Africa (5.01%, 95% CI: 4.43 to 66.3), Americas (9.30%, 95% CI: 6.40 to 13.3), USA and Latin Americas (7.10%, 95% CI: 6.93 to 28.3), Arabian Gulf (9.36%, 95% CI: 1.80 to 36.3), Asia (31.00%, 95% CI: 29.76 to 32.3), China (6.89%, 95% CI: 6.87 to 91.3), East Africa (6.03%, 95% CI: 5.96 to 9.3), EMRO (34.20%, 95% CI: 31.73 to 36.3), Europe-Africa-Asia (16.50%, 95% CI: 15.66 to 17.3), Europe-Africa-Asia-America-Australia (17.70%, 95% CI: 17.50 to 17.3), Europe-Africa-Asia-Americas (26.00%, 95% CI: 25.97 to 26.3), Europe-Asia-America-Australia (12.47%, 95% CI: 12.42 to 12.3), Europe-Asia-Americas (20.30%, 95% CI: 19.25 to 21.3), Europe-Asia-Americas-Oceania (88.60%, 95% CI: 78.28 to 94.3), and global (2.30%, 95% CI: 2.24 to 36.3).
Fig. 2. A forest plot of Distribution and prevalence of hepatotropic viruses across diverse geographical regions (a) and assessment of Heterogeneity in Prevalence Rates (b).
Note that no data pooling was performed for subgroups with only one study in its category, resulting in between-study and other heterogeneity statistics being reported as 0 and p-value of 1. However, each subgroup contributed to the overall effect of the study.
Рис. 2. Форест-график распределения и распространенности гепатотропных вирусов в различных географических регионах (a) и оценка неоднородности показателей распространенности (б).
Примечание: объединение данных не проводилось для подгрупп с единственным исследованием в своей категории, в результате чего различия между исследованиями и другая статистика гетерогенности данных были представлены как 0 и p = 1. Однако каждая подгруппа по-прежнему вносила вклад в общий эффект исследования.
Assessment of Heterogeneity in Prevalence Rates (Fig. 2 b): The Q-value, a metric assessing overall heterogeneity, showed a notable outcome (Q = 6486740.461, df = 32, p < 0.001). The I² statistic, reflecting the extent of authentic heterogeneity, was observed to be around 99.95%. Furthermore, the calculated tau squared (τ2) value, capturing the variance among studies, was determined as 1.5679.
Subgroup analysis by types (Fig. 3 a): HAV (82.5%, 95% CI: 45.2 to 96.4), HBV (8.6%, 95% CI: 6.3 to 11.7), HCV (15.1%, 95% CI: 12.8 to 17.8), HDV (8.9%, 95% CI: 1.7 to 36.0), HEV (13.9%, 95% CI: 10.9 to 17.6), dual HBV and HCV coinfection (2.2%, 95% CI: 0.6 to 8.0).
Fig. 3. Forest plot of: Subgroup analysis by Virus types (a); Composite prevalence rates of HBV infection among different risk groups (b); Prevalence of HBV varied across different continents (c); Prevalence of HCV among populations at risk (d); Regional prevalence of HCV (e).
Note that no data pooling was performed for subgroups with only one study in its category, resulting in between-study and other heterogeneity statistics being reported as 0 and I-value of 1. However, each subgroup still contributed to the overall effect of the study.
Рис. 3. Форест-график: анализ подгрупп по типам вируса (a); совокупные показатели распространенности инфекции HBV среди разных групп риска (б); различия распространенности HBV на разных континентах (в); распространенность HCV среди групп риска (г); региональная распространенность HCV (д).
Примечание: объединение данных не проводилось для подгрупп с единственным исследованием в своей категории, в результате чего статистические данные между исследованиями и другие показатели гетерогенности были представлены как 0, а p = 1. Однако каждая подгруппа по-прежнему вносила вклад в общий эффект исследования.
Composite prevalence rates of HBV infection among different risk groups (Fig. 3 b): General population (8.3%, 95% CI: 6.2 to 11.1), healthcare workers (4.0%, 95% CI: 1.3 to 11.2), hemodialysis patients (9.0%, 95% CI: 9.3 to 10.5), immigrants and refugees (7.2%, 95% CI: 7.1 to 7.3), low-risk patients (13.6%, 95% CI: 13.2 to 14.0), people with mental illness (84.0%, 95% CI: 83.2 to 84.8), pregnant women (4.8%, 95% CI: 0.4 to 41.6), PLWH (10.2%, 95% CI: 8.7 to 12.0), populations at risk (10.3%, 95% CI: 10.2 to 10.5).
Prevalence of HBV varied across different continents (Fig. 3 c): Africa (9.2%, 95% CI: 2.0 to 33.4%), Ethiopia (6.0%, 95% CI: 5.0 to 7.2), Nigeria (11.4%, 95% CI: 8.0 to 16.1), Somalia (9.1%, 95% CI: 3.1 to 24.4), Sudan (9.1%, 95% CI: 8.4 to 9.9), Africa-Asia (15.0%, 95% CI: 13.7 to 16.4), China (6.9%, 95% CI: 6.9 to 6.9), India (2.3%, 95% CI: 1.2 to 4.6), Thailand (5.1%, 95% CI: 5.06 to 14.3), Europe-Africa (7.8%, 95% CI: 6.7 to 9.0), Europe-Asia (84.0%, 95% CI: 83.2 to 84.8), global (2.3%, 95% CI: 2.2 to 2.4), Gulf regions (1.7%, 95% CI: 1.65 to 69.3), Iran (1.0%, 95% CI: 0.9 to 1.1).
Prevalence of HCV among populations at risk (Fig. 3 d): Healthcare Workers (HCWs) (5.58%, 95% CI: 5.02 to 6.19), low-risk individuals (2.6%, 95% CI: 1.30 to 5.30), PLWH (4.8%, 95% CI: 5.02 to 6.19), hemodialysis patients (34.8%, 95% CI: 27.90 to 7.80), homeless individuals (20.8%, 95% CI: 19.20 to 21.4), intermediate-risk individuals (11.8%, 95% CI: 2.70 to 39.50), people with mental illness (92.8%, 95% CI: 91.6 to 96.4), pregnant women (34.8%, 95% CI: 33.70 to 34.30), prisoners (23.8%, 95% CI: 17.7 to 30.7), special populations (35.8%, 95% CI: 23.1 to 49.1), waste pickers (8.8%, 95% CI: 7.1 to 9.1), populations at risk (35.8%, 95% CI: 5.02 to 6.19), high-risk individuals (28.3%, 95% CI: 15.3 to 46.0).
Regional prevalence of HCV (Fig. 3 e): Africa (7.42%, 95% CI: 3.00 to 17.18), Cameroon (17.55%, 95% CI: 1.21 to 78.71), Ethiopia (3.10%, 95% CI: 2.98 to 3.22), Ghana (3.00%, 95% CI: 2.90 to 3.11), Somalia (48.40%, 95% CI: 47.16 to 49.64), Sudan (2.00%, 95% CI: 1.73 to 2.32), Asia (11.20%, 95% CI: 10.68 to 11.74), Arabian Gulf countries (9.36%, 95% CI: 1.80 to 36.78), Europe-America-Asia-Americas (26.00%, 95% CI: 25.97 to 26.03), Europe-Africa-Asia-America-Australia (17.70%, 95% CI: 17.50 to 17.90), Europe-Asia (20.30%, 95% CI: 19.25 to 21.39), Europe-Asia-Americas-Oceania (92.00%, 95% CI: 91.55 to 92.43), Middle East (25.30%, 95% CI: 24.73 to 25.88).
In nearly all data pooling, the applied heterogeneity index I2 presented a value of either 0% (in the case of one meta-analysis) or about 99% in the case of more than one meta-analysis for the studied indicator. The same scenario was observed for other used heterogeneity and variability indices (Tau squared (τ2) and Q-statistic).
Discussion
Understanding the spread and prevalence of hepatotropic viruses across diverse geographic regions is paramount for formulating effective public health strategies. Our study was designed with the overarching goal of systematically synthesizing information from various meta-analyses to yield comprehensive insights into the intricate patterns of hepatotropic virus spread and prevalence.
The analysis unveiled substantial heterogeneity among included studies, as evidenced by the significant Q-statistic (Q = 6486740.461, df = 32, p < 0.001). The I2 statistic indicated that around 99.95% of the total variation was attributed to genuine heterogeneity among studies, reflecting notable differences in prevalence rates. This heterogeneity was further confirmed by the estimated tau squared (τ2) value of 1.5679, highlighting substantial between-study variance.These findings warrant adopting a mixed-effects statistical approach to enhance the precision and reliability of prevalence estimates.
These estimates present an extensive exploration into the intricate distribution and prevalence patterns of hepatotropic viruses across diverse geographical regions, shedding light on the multifaceted factors that contribute to the varying rates of prevalence. The observed disparities in prevalence are a result of a complex interplay of variables including healthcare infrastructure, demographic dynamics and healthcare practices. The study unearths a notably higher prevalence of hepatotropic viruses in the MENA region, where rates surge to 29.2%. This pronounced prevalence can be attributed to formidable obstacles in healthcare access and the complex challenges faced in executing effective public health efforts within the region. In contrast, North America and Europe exhibit comparatively lower prevalence rates ranging from 2.30% to 26.00%, underscoring the success of robust public health measures and advanced medical systems in curbing viral prevalence. The prevalence rates in African nations such as Somalia (49.46%) and Sudan (4.33%) reflect a distinct set of challenges including limited disease monitoring capacities, scarce healthcare resources and inadequate vaccination coverage, all further compounded by socioeconomic adversities and ongoing conflicts.
A comparative analysis of this study with existing research serves to highlight its distinctive contributions. The notably higher prevalence of HAV at 82.5% in this study surpasses previously reported rates of 31.4% and 52% in [67] and [68], possibly attributable to factors like water and food contamination, thereby emphasizing the critical role of elevated sanitation standards. HBV prevalence, notably lower at 8.6%, aligns with global figures (5.8%), underscoring the success of vaccination initiatives [69]. In the African context HBV prevalence varies between 9.2% and 11.4%, encompassing Ethiopia at 6.0% and Nigeria at 11.4. A trend correlates with the findings of [70] in Kenya, who reported a prevalence of 7.8%, Yendewa [71] in Sierra Leone with a prevalence of 13.0%, and Olaru[69] in the WHO African Region with a prevalence of 7.8%. The persistent concern of HCV prevalence (15.1%), transmitted primarily through blood contact, also finds reinforcement in Olaru’s [69] recent meta-analysis (10.3%), underscoring the critical importance of robust blood screening, strategic harm reduction strategies and the facilitation of accessible treatment options. The prevalence of HDV (8.9%) and HEV (13.9%), although relatively lower than certain reported figures, remains intertwined with inadequate sanitation and contaminated water sources, with consideration given to identification of increasing number of domestic and wild animal cases as reported by [69], thereby highlighting the multifaceted challenges in healthcare accessibility. Isolates of HEV from animals and sporadic human cases belong to genotype 4 in China and Vietnam which were part of endemic regions. In India, another endemic region, animal isolates all were members of genotype 4 and human isolates belonged to genotype 1 [69]. Furthermore, the prevalence of HDV (8.9%), often accompanying HBV co-infection, and HEV prevalence (13.9%), although lower than figures (35.8% and 21%) from [72] and [73] respectively, are linked to inadequate sanitation and contaminated water sources, thereby highlighting challenges in healthcare accessibility. Notably, the HDV prevalence aligns with the global occurrence range of 8.7% to 18.7% [2]. The relatively modest prevalence (2.2%) of dual HBV and HCV carriage can be attributed to specific risk factors including blood transfusions, substance abuse and high-risk sexual behaviors. The waning prevalence of HDV infection globally can be primarily attributed to the widespread implementation of HBV vaccination programs. These initiatives not only shrink the pool of susceptible HBsAg carriers but also showcase potential cross-protection against other hepatitis viruses, underscoring the interconnected nature of hepatitis virus vaccines [74].
The study delves even deeper into the prevalence across diverse population groups, intricately unraveling the interplay of risk factors, healthcare accessibility, and social determinants that collectively shape disease burden within each subgroup. The observed lower prevalence among low-risk groups like Healthcare Workers (HCWs) and individuals living with HIV (PLWH) serves to underscore the efficacy of well-implemented infection control measures and impactful awareness campaigns. HCWs, operating within stringent protective protocols, and PLWH, benefiting from ongoing healthcare engagements, exemplify the tangible dividends of proactive interventions. In contrast, high-risk groups manifest higher prevalence rates, a reflection of the complexities entrenched within their circumstances. Haemodialysis patients, subjected to frequent medical procedures, exhibit heightened risk due to their extensive exposure to bloodborne pathogens. This aligns seamlessly with findings by other researchers, such as the documented prevalence of 57.0% among People Who Inject Drugs (PWID) [75]. This study’s findings align with those of recent meta-analytic research on HBV prevalence, such as Vincent’s [76] 7.4% rate in the high-risk adult population and Wu’s 4.8% [72] prevalent rate in pregnant women, suggesting consistent distribution patterns
The heightened vulnerabilities of homeless individuals come to the forefront, as they are at especially high risk due to inadequate hygiene standards and limited healthcare access. The prevalence among individuals with serious mental illness gains significance, attributed to barriers rooted in stigma and adherence challenges to preventive measures. The elevated prevalence among pregnant women is linked to immunological changes inherent to pregnancy. Prison inmates, confined within close quarters with limited healthcare access, encounter heightened risk due to their constant close proximity. Distinct populations such as migrants, refugees and marginalized communities confront unique challenges that amplify their burden. Waste pickers, engaged in occupations with potential exposure to contaminated materials, face an augmented risk. Overall, the cumulative prevalence rate of 35.8% and 21% reported by [72] and [73] reflects the cumulative impact of diverse risk factors and determinants intricately woven into the population’s fabric.
The insights from this study extend even further when viewed through the lens of global variability in HCV prevalence. Categorizing prevalence rates by continent, the study unearths the intricate distribution dynamics and disparities that underpin disease prevalence. In Africa, the prevalence of HCV spans a spectrum between 3.00% and 17.18%, mirroring the disparities in healthcare infrastructure, access to preventive measures, socioeconomic conditions and cultural practices. The high prevalence of 17.55% in Cameroon highlights a substantial regional burden, whereas Ethiopia and Ghana exhibit more moderate burdens. In contrast, Somalia’s staggering prevalence of 48.40% sheds light on the unique challenges entrenched within specific regions. Across Asia, a sweeping variance in prevalence rates is evident. Afghanistan’s prevalence of 9.14% signifies diverse national dynamics intertwined with healthcare access and varying risk behaviors. The broader Asian landscape, boasting a prevalence of 31.00%, signifies a substantial burden, exemplified by Iran’s prevalence of 29.02%, emblematic of a pressing health challenge. These observations resonate harmoniously with Aghaei’s reported prevalence of 44.82% for the EMRO region [77]. The Americas, on the other hand, showcase a range of prevalence rates, with an overall prevalence of 11.20% and the Arabian Gulf displaying a prevalence of 9.36%. This mirrors the inherent diversity in healthcare systems and risk profiles. Interestingly, these prevalence rates align closely with Aghaei’s [77] reported prevalence among people who inject drugs in the Eastern Mediterranean region. The Europe-America-Asia-Americas region paints a prevalence of 26.00%, closely aligned with Olaru’s [69] reported 17.5% prevalence in the WHO European region. These shared figures underline interconnected burdens. Meanwhile, Europe-Africa-Asia-America-Australia showcases a prevalence of 17.70%, and Europe-Africa-Asia-Americas stands at 26.00%, echoing Aghaei’s [77] reported 18.86% in Asia, illustrating the constant change of healthcare infrastructure and practices. The strikingly high prevalence of 92.00% in Europe-Asia-Americas-Oceania necessitates targeted interventions due to the specific regional dynamics. The prevalence of 25.30% in the Middle East accentuates the necessity for bespoke interventions considering the unique challenges embedded in the region. The observation of a higher burden of HCV compared to HBV in pregnant women opens a fascinating avenue for exploration. This disparity could be attributed to differential transmission modes, regional prevalence variations, vertical transmission dynamics, effectiveness of prenatal screening, awareness campaigns and distinct immune responses. Such nuanced understanding guides interventions tailored to mitigate the impact of both viruses on pregnant women and their infants.
These findings not only underscore the urgency of tailored public health strategies, but also advocate for a comprehensive approach that encompasses improved healthcare access, proactive vaccination campaigns, strengthened sanitation infrastructure and heightened awareness initiatives. The profound global burden of hepatotropic viruses necessitates a continuous research agenda and sustained vigilance to effectively mitigate their prevalence. The study emphasizes that concerted actions are indispensable to not only address the immediate impacts of these viruses, but also to forge ahead in terms of prevention and control, all while acknowledging the nuanced contexts in which they thrive.
While this study undoubtedly contributes significantly, there is still a lot to be discovered about hepatotropic viruses. To improve the reliability and quality of evidence, future research endeavors should prioritize the establishment of well-defined protocols, designed to address potential methodological uncertainties. A more thorough study of the specific risk factors within different regions and a meticulous assessment of the impact of varying interventions would provide a more nuanced understanding of hepatotropic virus prevalence. A more profound exploration of the factors underpinning the divergent prevalence rates of Hepatitis B virus across continents could unravel crucial insights for tailoring region-specific interventions. Similarly, delving into the reasons behind the lower prevalence rates of hepatotropic viruses in certain regions, such as the Gulf regions and Iran, could offer valuable insight into the effectiveness of healthcare systems, comprehensive vaccination programs and awareness campaigns. This expansive research landscape offers a promising platform for generating not only a more dependable and comprehensive understanding of hepatotropic virus prevalence but also actionable insights for policymakers and healthcare professionals striving to effectively address the global burden of these viruses with precision.
While the study boasts a panoramic view of hepatotropic virus prevalence across diverse regions, certain limitations require acknowledgment. The heavy reliance on previously published articles and observational data could potentially compromise the transparency and reproducibility of the study. Furthermore, the methodology employed, which curtails the in-depth analysis of individual sample data, infection markers used, etc. could bring about discrepancies and inherent biases into the outcomes.
As observed in our results, the high heterogeneity in our meta-analyses with I2 values around 99% when data was pooled from multiple meta studies, likely stems from the combination of data on incidence by virus types from different regions. This naturally introduces significant variability, driven by geographical differences, healthcare practices, diagnostic methods and population demographics. Additional factors such as study design, time periods and sampling methods further contribute to the variation. The Q-statistic and τ2 confirm substantial between-study variance. While the random-effects model accounts for this, the high heterogeneity may limit the generalizability of our findings across regions with distinct epidemiological profiles.
While these pathogens share a tropism for the liver, the modes of transmission, risk factors for acquisition, natural history and the availability of vaccine are markedly different. The study does not differentiate active versus resolved. Even with these limitations, this study offers preliminary information that are valuable in the field and may herald for further inquiries into relative analysis. The approach of gathering the pathogen data into a monolith of information in a meta-meta-analysis may be of interest to the scientific community. Furthermore, the inclusion of studies from varied geographical regions enriches the study’s scope but also brings in variations in patient care practices, warranting careful interpretation.
To improve the reliability and quality of evidence, future research should prioritize the integration of well-structured studies, encompassing both the current meta-analysis and previously underappreciated published studies.
Conclusion
In conclusion, our study resonates with the broader aim of propelling global public health strategies and contributing to the WHO goal of eliminating viral hepatitis by the year 2030. This study offers intricate insights into the spread and prevalence of hepatotropic viruses across diverse regions, revealing complex factors shaping them. Varied prevalence rates mirror multifaceted transmission dynamics, including healthcare infrastructure, demographics, and practices. Notably, MENA faces higher rates, possibly due to healthcare challenges, while North America and Europe show lower rates due to robust measures. African nations like Somalia and Sudan struggle with several burdens from limited surveillance, healthcare and socioeconomics. Continent-based analysis uncovers nuanced global patterns, underlining the necessity for tailored public health strategies. This holistic understanding emphasizes healthcare access, vaccination, sanitation and awareness campaigns to mitigate hepatotropic virus burdens collectively and foster global health.
Об авторах
Shuaibu Suleiman Adeiza
Университет Ахмаду Белло; Усману Дафодийо Университет
Автор, ответственный за переписку.
Email: suleykestler2@gmail.com
ORCID iD: 0000-0002-9293-2600
доктор философии (PhD), руководитель лаборатории клинической практики, кафедра фармацевтической микробиологии и биотехнологии, факультет фармацевтических наук, кафедра клинической фармации и фармацевтической практики, факультет фармацевтических наук
Нигерия, Зария; СокотоMd. Aminul Islam
Медицинский колледж президента Абдула Хамида; Университет науки и технологий Ноакхали
Email: aminul@pahmc.edu.bd
ORCID iD: 0000-0003-1091-9726
магистр наук (MSc), докторант по клеточной и молекулярной биологии, продвинутая молекулярная лаборатория, кафедра микробиологии, лаборатория диагностики COVID-19, кафедра микробиологии
Бангладеш, Каримгандж, Кишорегандж, 2310; Ноакхали, 3814Hauwa’u Umar Mungadi
Усману Данфодио Университет
Email: hauwaumarmng@gmail.com
ORCID iD: 0000-0001-7200-1035
доктор философии (PhD), заведующий кафедрой, кафедра ветеринарной медицины, факультет ветеринарной медицины
Нигерия, СокотоAbdulmalik Bello Shuaibu
Усману Данфодио Университет
Email: abdulmalik.shuaibu@udusok.edu.ng
ORCID iD: 0000-0001-7684-2472
докторант (студент), руководитель лабораторного отдела вирусологии, кафедра ветеринарной микробиологии, факультет ветеринарной медицины
Нигерия, СокотоRanjit Sah
Институт медицины; Медицинский колледж, больница и научно-исследовательский центр Д.Д. Патиля, Университет Д.Д. Патиля
Email: ranjitsah@iom.edu.np
ORCID iD: 0000-0002-2695-8714
доктор философии (PhD) и руководитель отдела тестирования на грипп и COVID-19 (Национальный центр по гриппу), кафедра микробиологии, Университетская больница Трибхуван, афедра микробиологии
Непал, Катманду, 44600; Пуна, 411018, Махараштра, ИндияСписок литературы
- Lopez-Scarim J., Nambiar S.M., Billerbeck E. Studying T cell responses to hepatotropic viruses in the liver microenvironment. Vaccines (Basel). 2023; 11(3): 681. https://doi.org/10.3390/vaccines11030681
- Devarbhavi H., Asrani S.K., Arab J.P., Nartey Y.A., Pose E., Kamath P.S. Global burden of liver disease: 2023 update. J. Hepatology. 2023; 79(2): 516–37. https://doi.org/10.1016/j.jhep.2023.03.017
- Hassnine A.A., Saber M.A., Fouad Y.M., Sarhan H., Elsayed M.M., Zaki Z.M., et al. Clinical study on the efficacy of hepatitis B vaccination in hepatitis C virus related chronic liver diseases in Egypt. Virus Res. 2023; 323: 198953. https://doi.org/10.1016/j.virusres.2022.198953
- Antoniou T., Pritlove C., Shearer D., Tadrous M., Shah H., Gomes T. Accessing hepatitis C direct acting antivirals among people living with hepatitis C: a qualitative study. Int. J. Equity Health. 2023; 22(1): 112. https://doi.org/10.1186/s12939-023-01924-4
- Abesig J., Chen Y., Wang H., Sompo F.M., Wu I.X.Y. Prevalence of viral hepatitis B in Ghana between 2015 and 2019: A systematic review and meta-analysis. PLoS One. 2020; 15(6): e0234348. https://doi.org/10.1371/journal.pone.0234348
- Adane T., Getawa S. The prevalence and associated factors of hepatitis B and C virus in hemodialysis patients in Africa: A systematic review and meta-analysis. PLoS One. 2021; 16(6): e0251570. https://doi.org/10.1371/journal.pone.0251570
- Alali A.A., Abo-Shehada M.N. Prevalence of Hepatitis B Virus infection in the Gulf Cooperation Council: a systematic review and meta-analysis. BMC Infect. Dis. 2022; 22(1): 819. https://doi.org/10.1186/s12879-022-07806-4
- Kenfack-Momo R., Kenmoe S., Takuissu G.R., Ebogo-Belobo J.T., Kengne-Ndé C., Mbaga D.S., et al. Epidemiology of hepatitis B virus and/or hepatitis C virus infections among people living with human immunodeficiency virus in Africa: A systematic review and meta-analysis. PLoS One. 2022; 17(5): e0269250. https://doi.org/10.1371/journal.pone.0269250
- Qashqari F.S. Seroprevalence of hepatitis E virus infection in Middle Eastern Countries: A systematic review and meta-analysis. Medicina (Kaunas). 2022; 58(7): 905. https://doi.org/10.3390/medicina58070905
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 2022; 7(5): 396–415. https://doi.org/10.1016/s2468-1253(21)00472-6
- Irekeola A.A., Ear E.N.S., Mohd Amin N.A.Z., Mustaffa N., Shueb R.H. Antivirals against HCV infection: the story thus far. J. Infect. Dev. Ctries. 2022; 16(2): 231–43. https://doi.org/10.3855/jidc.14485
- Adeiza SS, Aminul I. Meta-meta-analysis of the mortality risk associated with MRSA compared to MSSA bacteraemia. Infez. Med. 2024;32(2):131. http://dx.doi.org/10.53854/liim-3202-2
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021; 10(1): 89. https://doi.org/10.1186/s13643-021-01626-4
- Christensen E. Quality of reporting of meta-analyses: the QUOROM statement. Will it help? J. Hepatol. 2001; 34(2): 342–5. https://doi.org/10.1016/s0168-8278(00)00002-7
- Suleiman A.S., Islam M.A., Akter M.S., Amin M.R., Werkneh A.A., Bhattacharya P. A meta-meta-analysis of co-infection, secondary infections, and antimicrobial resistance in COVID-19 patients. J. Infect. Public Health. 2023; 16(10): 1562–90. https://doi.org/10.1016/j.jiph.2023.07.005
- Shea B.J., Hamel C., Wells G.A., Bouter L.M., Kristjansson E., Grimshaw J., et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009; 62(10): 1013–20. https://doi.org/10.1016/j.jclinepi.2008.10.009
- Adeiza S.S., Shuaibu A.B., Shuaibu G.M. Random effects meta-analysis of COVID-19/S. aureus partnership in co-infection. GMS Hyg. Infect. Control. 2020; 15: Doc29. https://doi.org/10.3205/dgkh000364
- Adeiza SS, Islam MA, Shittu A. Global, regional, and national burdens: An overlapping meta-analysis on Staphylococcus aureus and its drug-resistant strains. One Health Bull. 2024:10-4103. https://doi.org/10.4103/ohbl.ohbl_10_24
- Borenstein M. In a meta-analysis, the I-squared statistic does not tell us how much the effect size varies. J. Clin. Epidemiol. 2022; 152: 281–4. https://doi.org/10.1016/j.jclinepi.2022.10.003
- Borenstein M. Comprehensive meta-analysis software. In: Systematic Reviews in Health Research: Meta-Analysis in Context. Wiley; 2022: 535–48.
- Agyeman A.A., Ofori-Asenso R. Prevalence of HIV and hepatitis B coinfection in Ghana: a systematic review and meta-analysis. AIDS Res Ther. 2016; 13: 23. https://doi.org/10.1186/s12981-016-0107-x
- Agyeman A.A., Ofori-Asenso R., Mprah A., Ashiagbor G. Epidemiology of hepatitis C virus in Ghana: a systematic review and meta-analysis. BMC Infect. Dis. 2016; 16: 391. https://doi.org/10.1186/s12879-016-1708-7
- Ahmad T., Hui J., Musa T.H., Behzadifar M., Baig M. Seroprevalence of hepatitis E virus infection in pregnant women: a systematic review and meta-analysis. Ann. Saudi Med. 2020; 40(2): 136–46. https://doi.org/10.5144/0256-4947.2020.136
- Ajuwon B.I., Yujuico I., Roper K., Richardson A., Sheel M., Lidbury B.A. Hepatitis B virus infection in Nigeria: a systematic review and meta-analysis of data published between 2010 and 2019. BMC Infect. Dis. 2021; 21(1): 1120. https://doi.org/10.1186/s12879-021-06800-6
- Alemu A.A., Zeleke L.B., Aynalem B.Y., Kassa G.M. Hepatitis B virus infection and its determinants among pregnant women in Ethiopia: a systematic review and meta-analysis. Infect. Dis. Obstet. Gynecol. 2020; 2020: 9418475. https://doi.org/10.1155/2020/9418475
- Alonso M., Gutzman A., Mazin R., Pinzon C.E., Reveiz L., Ghidinelli M. Hepatitis C in key populations in Latin America and the Caribbean: systematic review and meta-analysis. Int. J. Public Health. 2015; 60(7): 789–98. https://doi.org/10.1007/s00038-015-0708-5
- Amini N., Alavian S.M., Kabir A., Aalaei-Andabili S.H., Saiedi Hosseini S.Y., Rizzetto M. Prevalence of hepatitis D in the Eastern Mediterranean region: systematic review and meta-analysis. Hepat. Mon. 2013; 13(1): e8210. https://doi.org/10.5812/hepatmon.8210
- Ashkani-Esfahani S., Alavian S.M., Salehi-Marzijarani M. Prevalence of hepatitis C virus infection among hemodialysis patients in the Middle-East: A systematic review and meta-analysis. World J. Gastroenterol. 2017; 23(1): 151. https://doi.org/10.3748/wjg.v23.i1.151
- Atlaw D., Sahiledengle B., Tariku Z. Hepatitis B and C virus infection among healthcare workers in Africa: a systematic review and meta-analysis. Environ. Health Prev. Med. 2021; 26(1): 61. https://doi.org/10.1186/s12199-021-00983-9
- Azami M., Hafezi Ahmadi M.R., Sayehmiri K. Hepatitis B vaccination efficacy in Iranian healthcare workers: a meta-analysis study. Hepat. Mon. 2017; 17(1): e37781. https://doi.org/10.5812/hepatmon.37781
- Badawi M.M., Atif M.S., Mustafa Y.Y. Systematic review and meta-analysis of HIV, HBV and HCV infection prevalence in Sudan. Virol. J. 2018; 15(1): 148. https://doi.org/10.1186/s12985-018-1060-1
- Batham A., Narula D., Toteja T., Sreenivas V., Puliyel J.M. Systematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatrics. 2007; 44(9): 663.
- Chemaitelly H., Mahmud S., Rahmani A.M., Abu-Raddad L.J. The epidemiology of hepatitis C virus in Afghanistan: systematic review and meta-analysis. Int. J. Infect. Dis. 2015; 40: 54–63. https://doi.org/10.1016/j.ijid.2015.09.011
- Chen H.Y., Shen D.T., Ji D.Z., Han P.C., Zhang W.M., Ma J.F., et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019; 68(3): 512–21. https://doi.org/10.1136/gutjnl-2018-316601
- Daw M.A., Daw A.M., Sifennasr N.E.M., Draha A.M., Daw A.M., Daw A.M., et al. The epidemiology of hepatitis D virus in North Africa: a systematic review and meta-analysis. ScientificWorldJournal. 2018; 2018: 9312650. https://doi.org/10.1155/2018/9312650
- Desikan P., Khan Z. Prevalence of hepatitis B and hepatitis C virus co-infection in India: a systematic review and meta-analysis. Indian J. Med. Microbiol. 2017; 35(3): 332–9. https://doi.org/10.4103/ijmm.ijmm_17_257
- Fernández Villalobos N.V., Kessel B., Rodiah I., Ott J.J., Lange B., Krause G. Seroprevalence of hepatitis E virus infection in the Americas: Estimates from a systematic review and meta-analysis. PLoS One. 2022; 17(6): e0269253. https://doi.org/10.1371/journal.pone.0269253
- Farajzadegan Z., Hoseini S.G., Kelishadi R., Jamshidi F., Nokhodian Z., Noori R., et al. Systematic review and meta-analysis on the age-specific seroprevalence of hepatitis A in Iran. J. Res. Med. Sci. 2014; 19(Suppl. 1): S56–63.
- Giri S., Sahoo S., Angadi S., Afzalpurkar S., Sundaram S., Bhrugumalla S. Seroprevalence of hepatitis B virus among pregnant women in India: A systematic review and meta-analysis. J. Clin. Exp. Hepatol. 2022; 12(6): 1408–19. https://doi.org/10.1016/j.jceh.2022.08.005
- Harfouche M., Chemaitelly H., Mahmud S., Chaabna K., Kouyoumjian S.P., Al Kanaani Z., et al. Epidemiology of hepatitis C virus among hemodialysis patients in the Middle East and North Africa: systematic syntheses, meta-analyses, and meta-regressions. Epidemiol. Infect. 2017; 145(15): 3243–63. https://doi.org/10.1017/s0950268817002242
- Hassan-Kadle M.A., Osman M.S., Ogurtsov P.P. Epidemiology of viral hepatitis in Somalia: Systematic review and meta-analysis study. World J. Gastroenterol. 2018; 24(34): 3927–57. https://doi.org/10.3748/wjg.v24.i34.3927
- Horvatits T., Ozga A.K., Westhölter D., Hartl J., Manthey C.F., Lütgehetmann M., et al. Hepatitis E seroprevalence in the Americas: A systematic review and meta-analysis. Liver Int. 2018; 38(11): 1951–64. https://doi.org/10.1111/liv.13859
- Hughes E., Bassi S., Gilbody S., Bland M., Martin F., et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry. 2016; 3(1): 40–8. https://doi.org/10.1016/s2215-0366(15)00357-0
- Kafeero H.M., Ndagire D., Ocama P., et al. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Arch. Public Health. 2021; 79(1): 167. https://doi.org/10.1186/s13690-021-00686-1
- Kouyoumjian S.P., Chemaitelly H., Abu-Raddad L.J. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci. Rep. 2018; 8(1): 1661. https://doi.org/10.1038/s41598-017-17936-4
- Larney S., Kopinski H., Beckwith C.G., Zaller N.D., Jarlais D.D., Hagan H., et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: Results of a systematic review and meta-analysis. Hepatology. 2013; 58(4): 1215–24. https://doi.org/10.1002/hep.26387
- Leroi C., Adam P., Khamduang W., Kawilapat S., Ngo-Giang-Huong N., Ongwandee S., et al. Prevalence of chronic hepatitis B virus infection in Thailand: a systematic review and meta-analysis. Int. J. Infect. Dis. 2016; 51: 36–43. https://doi.org/10.1016/j.ijid.2016.08.017
- Leumi S., Bigna J.J., Amougou M.A., Ngouo A., Nyaga U.F., Noubiap J.J. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin. Infect. Dis. 2020; 71(11): 2799–806. https://doi.org/10.1093/cid/ciz1170
- Li P., Liu J., Li Y., Su J., Ma Z., Bramer W.M., et al. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 2020; 40(7): 1516–28. https://doi.org/10.1111/liv.14468
- Mahamat G., Kenmoe S., Akazong E.W., Ebogo-Belobo J.T., Mbaga D.S., Bowo-Ngandji A., et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J. Hepatol. 2021; 13(9): 1190–202. https://doi.org/10.4254/wjh.v13.i9.1190
- Mahmud S., Akbarzadeh V., Abu-Raddad L.J. The epidemiology of hepatitis C virus in Iran: Systematic review and meta-analyses. Sci. Rep. 2018; 8(1): 150. https://doi.org/10.1038/s41598-017-18296-9
- Moghaddasifar I, B. Lankarani K, Moosazadeh M, et al. Prevalence of hepatitis B virus infection among pregnant women in Iran: A systematic review and meta-analysis. Iran. J. Cancer Prev. 2016; 9(6). https://doi.org/10.17795/ijcp-3703
- Mohamoud Y.A., Riome S., Abu-Raddad L.J. Epidemiology of hepatitis C virus in the Arabian Gulf countries: Systematic review and meta-analysis of prevalence. Int. J. Infect. Dis. 2016; 46: 116–25. https://doi.org/10.1016/j.ijid.2016.03.012
- Mora N., Adams W.H., Kliethermes S., Dugas L., Balasubramanian N., Sandhu J., et al. A synthesis of hepatitis C prevalence estimates in Sub-Saharan Africa: 2000–2013. BMC Infect. Dis. 2016; 16: 283. https://doi.org/10.1186/s12879-016-1584-1
- Musa B., Bussell S., Borodo M., Samaila A.A., Femi O.L. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger. J. Clin. Pract. 2015; 18(2): 163–72. https://doi.org/10.4103/1119-3077.151035
- Ofori-Asenso R., Agyeman A.A. Hepatitis B in Ghana: a systematic review & meta-analysis of prevalence studies (1995–2015). BMC Infect. Dis. 2016; 16: 130. https://doi.org/10.1186/s12879-016-1467-5
- Rao V.B., Johari N., Du Cros P., Messina J., Ford N., Cooke G.S. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 2015; 15(7): 819–24. https://doi.org/10.1016/s1473-3099(15)00006-7
- Rossi C., Shrier I., Marshall L., Cnossen S., Schwartzman K., Klein M.B., et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One. 2012; 7(9): e44611. https://doi.org/10.1371/journal.pone.0044611
- Salari N., Darvishi N., Hemmati M., Shohaimi S., Ghyasi Y., Hossaini F., et al. Global prevalence of hepatitis C in prisoners: a comprehensive systematic review and meta-analysis. Arch. Virol. 2022; 167(4): 1025–39. https://doi.org/10.1007/s00705-022-05382-1
- Salehi-Vaziri M., Sadeghi F., Almasi Hashiani A., Gholami Fesharaki M., Alavian S.M. Hepatitis B virus infection in the general population of Iran: an updated systematic review and meta-analysis. Hepat. Mon. 2016; 16(4): e35577. https://doi.org/10.5812/hepatmon.35577
- Souza-Silva G., Zolnikov T.R., Ortolani P.L., Cruvinel V.R.N., Dias S.M., Mol M.P.G. Hepatitis B and C prevalence in waste pickers: a global meta-analysis. J. Public Health (Oxf.). 2022; 44(4): 761–9. https://doi.org/10.1093/pubmed/fdab285
- Stockdale A.J., Kreuels B., Henrion M.Y.R., Giorgi E., Kyomuhangi I., de Martel C., et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020; 73(3): 523–32. https://doi.org/10.1016/j.jhep.2020.04.008
- Wang H., Men P., Xiao Y., Gao P., Lv M., Yuan Q., et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect. Dis. 2019; 19(1): 811. https://doi.org/10.1186/s12879-019-4428-y
- Yazie T.D., Tebeje M.G. An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infect. Dis. 2019; 19(1): 917. https://doi.org/10.1186/s12879-019-4486-1
- Azevedo T.C.L., Zwahlen M., Rauch A., Egger M., Wandeler G. Hepatitis C in HIV-infected individuals: a systematic review and meta-analysis of estimated prevalence in Africa. J. Int. AIDS Soc. 2016; 19(1): 20711. https://doi.org/10.7448/ias.19.1.20711
- Li P., Ji Y., Li Y., Ma Z., Pan Q. Estimating the global prevalence of hepatitis E virus in swine and pork products. One Health. 2022; 14: 100362. https://doi.org/10.1016/j.onehlt.2021.100362
- Takuissu G.R., Kenmoe S., Amougou Atsama M., Atenguena Okobalemba E., Mbaga D.S., Ebogo-Belobo J.T., et al. Global epidemiology of occult hepatitis B virus infections in blood donors, a systematic review and meta-analysis. PLoS One. 2022; 17(8): e0272920. https://doi.org/10.1371/journal.pone.0272920
- Hofmeister M.G., Xing J., Foster M.A., Augustine R.J., Burkholder C., Collins J., et al. Hepatitis A person-to-person outbreaks: epidemiology, morbidity burden, and factors associated with hospitalization—multiple states, 2016–2019. J. Infect. Dis. 2021; 223(3): 426–34. https://doi.org/10.1093/infdis/jiaa636
- Olaru I.D., Beliz Meier M., Mirzayev F., Prodanovic N., Kitchen P.J., Schumacher S.G., et al. Global prevalence of hepatitis B or hepatitis C infection among patients with tuberculosis disease: systematic review and meta-analysis. EClinicalMedicine. 2023; 58: 101938. https://doi.org/10.1016/j.eclinm.2023.101938
- Makokha G.N., Zhang P., Hayes C.N., Songok E., Chayama K. The burden of Hepatitis B virus infection in Kenya: A systematic review and meta-analysis. Front. Public Health. 2023; 11: 986020. https://doi.org/10.3389/fpubh.2023.986020
- Yendewa G.A., Wang G.M., James P.B., Massaquoi S.P.E., Yendewa S.A., Ghazawi M., et al. Prevalence of chronic hepatitis B virus infection in Sierra Leone, 1997–2022: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2023; 109(1): 105–14. https://doi.org/10.4269/ajtmh.22-0711
- Qiu L.X., Huang Y., Quan J.L., Bi Z.F., Zhong G.H., Wang J.Y., et al. Prognosis of hepatitis E infection in patients with chronic liver disease: A meta-analysis. J. Viral Hepat. 2023; 30(2): 101–7. https://doi.org/10.1111/jvh.13754
- Raji Y.E., Toung O.P., Taib N.M., Sekawi Z.B. Meta-analysis and moderator analysis of the seroprevalence of hepatitis E in South-Eastern Asia. Sci. Rep. 2023; 13(1): 11880. https://doi.org/10.1038/s41598-023-37941-0
- Asselah T., Rizzetto M. Hepatitis D virus infection. N. Engl. J. Med. 2023; 389(1): 58–70. https://doi.org/10.1056/nejmra2212151
- Magri M.C., Manchiero C., Dantas B.P., da Silva Nunes A.K., Vaz Gago Prata T., Domingos D.E.A., et al. Hepatitis C among people who inject drugs (PWID) in Latin America and the Caribbean: A meta-analysis of prevalence over three decades. J. Stud. Alcohol Drugs. 2023; 84(1): 118–27. https://doi.org/10.15288/jsad.22-00014
- Vincent J.P., Nyamasege C., Wang S., Madec Y., Shimakawa Y. Prevalence of hepatitis B, C, and D virus infection in Haiti: A systematic review and meta-analysis. Front. Public Health. 2023; 10: 1099571. https://doi.org/10.3389/fpubh.2022.1099571
- Aghaei A.M., Gholami J., Sangchooli A., Rostam-Abadi Y., Olamazadeh S., Ardeshir M., et al. Prevalence of injecting drug use and HIV, hepatitis B, and hepatitis C in people who inject drugs in the Eastern Mediterranean region: a systematic review and meta-analysis. Lancet Glob Health. 2023; 11(8): e1225–37. https://doi.org/10.1016/s2214-109x(23)00267-x
Дополнительные файлы