Частота выявления антител к вирусу Западного Нила среди пациентов с острой фебрильностью в Илорине, Нигерия
- Авторы: Odebisi-Omokanye M.B.1, Suleiman M.M.2, Sulaiman M.K.1, Atolagbe S.A.1
-
Учреждения:
- Университет Илорина
- Саммитский университет Оффа
- Выпуск: Том 69, № 4 (2024)
- Страницы: 320-328
- Раздел: ОРИГИНАЛЬНЫЕ ИССЛЕДОВАНИЯ
- URL: https://virusjour.crie.ru/jour/article/view/16646
- DOI: https://doi.org/10.36233/0507-4088-241
- EDN: https://elibrary.ru/pvhwlc
- ID: 16646
Цитировать
Полный текст
Аннотация
Введение. Вирус Западного Нила (ВЗН), представитель семейства Flaviviridae, является одним из наиболее широко распространенных арбовирусов в мире. В развивающихся странах, таких как Нигерия, лихорадку, возникающую в результате инфекции ВЗН, часто ошибочно ассоциируют с малярией или брюшным тифом из-за неправильного диагноза и низкого уровня осведомленности об этой инфекции. В настоящем исследовании определяли распространенность антител к ВЗН классов IgM и IgG среди пациентов с лихорадкой в мегаполисе Илорин.
Материалы и методы. В общей сложности у 200 пациентов были взяты образцы крови, и каждая сыворотка была проверена на наличие антител к ВЗН классов IgM и IgG с использованием непрямого иммуноферментного анализа (ELISA). Были рассчитаны статистическая корреляция и коэффициенты логистической регрессии.
Pезультаты. В целом анти-ВЗН IgM были выявлены в 6% (12/200) пациентов с острой лихорадкой, с более высокой частотой выявления (6,30%) у женщин, чем у мужчин (5,45%).Частота выявления анти-ВЗН IgG составила 52% (104/200), при этом серопревалентность среди мужчин составила 50,67%, а среди женщин ‒ 38,95%. На стадии реконвалесценции, о которой свидетельствует одновременное обнаружение антител к ВЗН классов IgG и IgM, находились 5,4% (11/200) участников. Была получена статистически значимая корреляция серопозитивности с возрастом и религией респондентов, тогда как пол, род занятий, использование противомоскитных сеток и уровень образования не имели положительной корреляции при p < 0,05. Однако на основе отношения рисков при 95% ДИ и коэффициентов логистической регрессии такие факторы риска, как переливание крови, место проживания, инфицирование малярийным плазмодием, близость к стоячей воде и зарослям кустарника, были значимыми для положительного результата выявления анти-ВЗН-IgG и -IgM.
Заключение. Результаты этого исследования показывают циркуляцию ВЗН в исследуемой зоне. Клиницистам/врачам необходимо срочно включить скрининг на вирус Западного Нила у пациентов с лихорадкой перед началом лечения.
Ключевые слова
Полный текст
Introduction
West Nile virus (WNV) is one of the widely distributed arboviruses globally, and a pathogen of public health significance in both humans and animals [1]. This mosquito-borne virus belongs to the genus Flavivirus within the family of Flaviviridae [2].
West Nile virus (WNV) was first isolated in 1937 in the West Nile district of Uganda while the first reported human epidemic occurred in Israel in 1951 [3]. Subsequently, it was found to cause outbreaks in Africa, the Middle East, and Western Asia [4]. There has been significant geographical spread of WNV worldwide, abetted by activities such as international travel, globalization and land use.
The main vectors of WNV are mosquitoes belonging to Culex spp and Aedes spp [5, 6]. The virus is maintained in nature by mosquito – bird – mosquito transmission cycle. Nonetheless, other modes of transmission have been recognized: breastfeeding, blood transfusion, organ transplantation, and occupational exposure among laboratory workers [4, 7, 8]. Humans and other vertebrates such as horses do not play a role in the transmission of WNV and are referred to as «dead-end» hosts. This is because they are susceptible but unable to transmit to mosquitoes due to the short-lived and low viremia [9]. They can however manifest severe disease or death as a result of the infection [10].
In humans, 80% of WNV infections are asymptomatic, 20% develop a flu-like illness, and less than 1% progress to develop neurological disease, which is predominant in the elderly and immunocompromised [11, 12]. In symptomatic individuals, symptoms such as mild fever, headaches, nausea, and/or rashes appear between 3‒14 days although in severe cases may be biphasic with symptoms sustained for up to 60 days [4, 13]. The often-non-specific symptoms of West Nile Virus infections in human makes differential neurological disease, which can be severe causing viral encephalitis, meningitis, and/or seizures [4, 13].
Differential diagnosis of WNV infection in humans is seemingly complicated due to the associated non-specific symptoms [14, 15]. In developing countries like Nigeria, this is even more difficult as a result of the unavailability of appropriate diagnostic infrastructure and low level of awareness of arboviruses leading to misdiagnosis and underdiagnosis. Owing to this, many cases of WNV infection are often regarded as fever of unknown cause resulting in wrong treatment which leads to high morbidity and mortality [16]. Further evidence also supports that the prevalence of WNV in Nigeria is sparse. Therefore, this study was conducted among patients with acute febrile conditions to elucidate the level of exposure to the virus and highlight the need for WNV routine testing among febrile patients before the commencement of treatment.
Materials and methods
Study design and population
This hospital-based cross-sectional study was conducted from May to July 2023 in selected health facilities of Ilorin City, Kwara state, Nigeria. Ilorin is a city in Kwara State situated at 80° 301 North, 4° 301 East, 300 km from Lagos, and 500 km from Abuja, the Federal Capital Territory. It is in the Guinea savannah vegetation zone, known to breed various mosquito species and with hot, dry, and wet-mild seasons that might vary yearly. Three primary and secondary health facilities were used as sampling locations for moderate representation of the populace.
Only consenting patients attending the selected hospitals and exhibiting fever symptoms (temperature above > 37.8 °C) were recruited for the study. Enrolled in the study were 200 (73 males and 127 females; age range: ≤ 10‒70 years) consenting in and out-patients presenting with fever regardless of age and gender. Excluded from the study were febrile patients who did not give their consent to be enrolled on the study.
Data and Sample Collection
Data collection was via a well-structured close-ended questionnaire. This was used to obtain the socio-demographic information of enrollees and predisposing factors to WNV. For sample collection, a total of 200 blood samples were collected across the locations after screening with the inclusion criteria. The collected venous blood sample was kept in a prelabelled plain sampling bottle to allow for clotting at room temperature, and serum separation was at 3000 rpm for 5 minutes. According to the manufacturer’s instructions (i.e. Sunlong Biotech, Co. Ltd., China) IgG and IgM screening for WNV was conducted on the sera. The reagents included the negative and positive controls, HRP-conjugate, Sample diluent, Chromogen Solution A, Chromogen Solution B, Stop and wash solution respectively. Samples were added to the Microplate wells and applied to the specific antigen. Horseradish Peroxidase (HRP)-conjugated antigen specific for WNV-IgG/IgM was added to each Microplate well and incubated for formation of antigen-antibody-Enzyme labeled antigen complex. The wash solution was used to remove any unbound reagent, then the TMB substrate solution was added to each well. This was followed by addition of stop solution where only those wells that contain WNV-IgG and HRP conjugated WNV antigen will appear blue in color and then turn yellow after the addition of the stop solution. The optical density (OD) was measured spectrophotometrically at wavelength of 450 nm while the qualitative determination of the respective immunoglobulin was determined by comparing with the cut-off value.
Result Interpretation for assay
The interpretation according to manufacturer instruction is based on the following standard (Sunlong Biotech LTD);
- Test effectiveness = average value of positive control ≥ 1.00 while the average value of negative control ≤ 0.10.
- The critical value (cut-off) calculation = the average value of negative control + 0.15.
- Negative judgement = if the OD value < cut-off value.
- Positive judgement = if the OD value ≥ cut-off value.
Ethical Consideration
The protocol for this study was submitted to and approved by the Ethics and Health Research Board of the Ministry of Health, Ilorin, Kwara State, Nigeria (ERC/MOH/2023/02/091). The protocol followed the guidelines of Helsinki Declaration for the use of human subjects. Before enrolment into the study and sample collection, informed consent was obtained from all the participants and from the parents and legal guardians (in case of participants who are less than 18 years old). Strict confidentiality was maintained as all results were not traceable to the patient identifiers and were kept anonymous. The inclusion of religion is for epidemiological completeness and not to imply causation or judgment.
Statistical Analysis
Data obtained from questionnaires were presented in tables and graphs. All statistical analysis was done using the Statistical Package for the Social Science (SPSS) version 27.0 software package. The significance of the values obtained was determined at p < 0.05 and 95% confidence interval to determine the relationship between the variable and the prevalence of the infection. Furthermore, logistic regression coefficients through Google Colab and Python programming language (version 3.11) were used for IgG and IgM positivity to the risk factors and the odd ratio.
Results
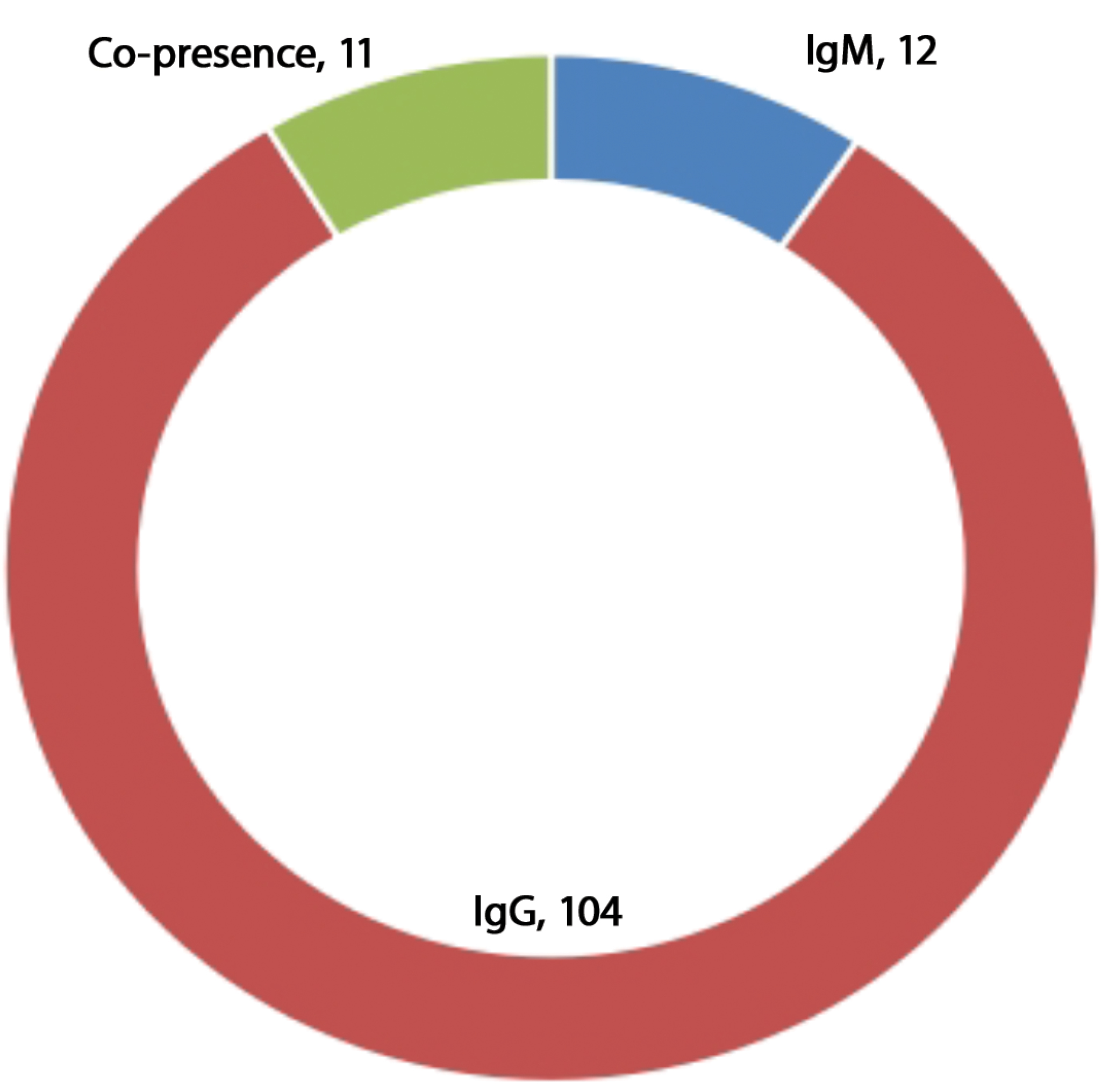
Of the 200 sera samples tested, 12 were positive for anti-WNV IgM and 104 for IgG antibody respectively. This accounts for 6% of recent/current infections and 52% of previous infections respectively among the febrile patients enrolled in the study. Additionally, 11 (5.5%) of the respondents were having an ongoing or recent immune activity due to co-detection of IgG and IgM antibodies (Fig. 1).
Fig. 1. Prevalence of antibodies to WNV among febrile respondents in Ilorin metropolis.
Рис. 1. Распространенность антител к ВЗН среди респондентов с лихорадкой в мегаполисе Илорин.
The mean age of the study participants was 28 years, while median and range is 15.5 years and 70 years respectively suggesting younger individuals are more frequently affected with fewer older outliers. There was no observed statistical significance (p > 0.05) in the presence of anti-WNV IgM antibodies with respect to age, gender, educational level and occupation (Table 1). The distribution of age group showed that subjects in the age group 11‒20 years had the highest prevalence of 26.5% (53/200) and 3.5% (7/200), followed by subjects in the age range 21‒30 years with the prevalence of 16.5% (33/200) and 1% (2/200), subjects in the age range 61‒70 years had lowest prevalence of 0% (0/200) and 0.5% (1/200) for anti-WNV IgG and IgM antibodies respectively (Table 1). However, the Odds Ratio (OR) for the anti-WNV-IgM positivity for different age groups is closer to 1 than that of IgG antibodies, but the p-value was statistically significant for IgG positivity.
Table 1. Relationship between demographic factors and distribution pattern of WNV IgM and IgG antibodies among febrile patients in selected hospitals in Ilorin, metropolis
Таблица 1. Связь между демографическими факторами и распределением антител классов IgM и IgG к вирусу Западного Нила среди пациентов с лихорадкой в отдельных больницах мегаполиса Илорин
Demographic Characteristics Демографические характеристики | n | IgM +Ve (%) | p-value OR | 95% CI Lower‒Upper | IgG +Ve (%) | p-value OR | 95% CI Lower‒Upper |
Gender / Пол | |||||||
Male / Мужской | 73 | 4 (5.45) | 0.856 0.930 | 0.270‒2.900 | 37 (50.68) | 0.800 0.729 | 0.482‒1.321 |
Female / Женский | 127 | 8 (6.30) | 67 (38.95) | ||||
Total / Всего | 200 | 12 (6) | 104 (57.1) | ||||
Age (Years) / Возраст (лет) | |||||||
≤ 10 | 43 | 2 (1) | 0.660 0.883 | 0.750‒1.602 | 33 (16.5) | 0.000* 0.602 | 0.198‒0.698 |
11‒20 | 91 | 7 (3.5) | 53 (26.5) | ||||
21‒30 | 48 | 1 (0.5) | 12 (6) | ||||
31‒40 | 4 | 1 (0.5) | 1 (0.55) | ||||
41‒50 | 9 | 1 (0.5) | 4 (2) | ||||
51‒60 | 3 | 0 (0) | 0 (0) | ||||
61‒70 | 2 | 0 (0) | 1 (0.5) | ||||
Total / Всего | 200 | 12 (6) | 104 (52) | ||||
Religion / Религия | |||||||
Islam / Ислам | 124 | 7 (3.5) | 0.781 0.814 | 0.249‒2.658 | 73 (36.5) | 0.008* 3.700 | 1.683‒4.604 |
Christianity / Христианство | 75 | 5 (2.5) | 31 (15.5) | ||||
Total / Всего | 200 | 12 (6) | 104 (52) | ||||
Formal Education / Образование | |||||||
Yes / Да | 125 | 8(4) | 0.864 2.976 | 0 | 66 (33) | 0.850 0.099 | 0.008‒1.280 |
No / Нет | 75 | 4(2) | 38 (19) | ||||
Total / Всего | 200 | 12(6) | 104 (52) | ||||
Occupation / Занятие | |||||||
Employed / Трудящиеся | 127 | 11(5.5) | 0.290 0 | 0 | 62 (31) | 0.140 2.650 | 0.850‒9.424 |
Unemployed / Безработные | 10 | 0(0) | 8 (4) | ||||
Student / Студент | 63 | 1(0.5) | 34 (17) | ||||
Total Всего | 200 | 12(6) | 104 (52) | ||||
Note. *Statistically significant (p < 0.05); OR ‒ odds ratio; CI ‒ Confidence Interval.
Примечание. *Статистически значимо (p < 0,05); OR ‒ отношение шансов; CI ‒ доверительный интервал.
The OR for IgM positivity among males compared to females is 0.930 (95% CI: 0.270‒2.900) indicating that the odds of being IgM antibody positive for males is 0.930 times lower than females while that of IgG antibodies is 0.729 (95% CI: 0.482‒1.321). For religion, OR IgM positivity among Christians compared to Muslims is 0.814 (95% CI: 0.249‒2.658) while 3.700 (95% CI: 1.683‒4.604) for IgG antibodies. Seropositivity was higher among Islam faith subjects with prevalence of 36.5% (73/200) and 3.5% (7/200) for anti-WNV IgG and IgM antibodies, while a lower prevalence was observed for the Christian faith subjects 15.5% (31/200) WNV IgG and 2.5% (5/200) WNV IgM. The result further shows higher odds of being IgG antibody positive amongst Christians and lower odds of being IgM antibody positive respectively compared to Muslim respondents. A higher odd of being IgM antibody positive amongst individuals with no formal education is recorded at 2.976 (95% CI: 0‒infinity) while lower odds for IgG positivity were recorded at 0.099 (95% CI: 0.008‒1.280). The odds ratio for unemployment in this study was 2.650 (95% CI: 0.850‒9.424).
The results were also analyzed in relation to some predisposing factors to the acquisition of WNV including; use of mosquito nets, blood transfusion, presence of malaria parasite, presence of typhoid fever, residential area, nearness of residence to bush, closeness to stagnant water and use of mosquito repellent at 1.397, 1.396, 0.765, 2.503, 0.981, 1.645, and 0.999 odds ratios respectively (Tables 2 and 3). The result showed no statistically significant relationship (p > 0.05) to the risk factors and the presence of anti-WNV IgM and IgG antibodies but with notable influence of the risk factors to anti-WNV IgG and IgM positivity via the odd ratio at 95% CI (Tables 2 and 3).
Table 2. Prevalence of anti-WNV IgG antibodies in relation to risk factors among febrile patients attending selected hospitals in the Ilorin metropolis
Таблица 2. Частота выявления антител класса IgG к вирусу Западного Нила в зависимости от факторов риска среди пациентов с лихорадкой в отдельных больницах мегаполиса Илорин
Risk Factors / Факторы риска | n | IgG +Ve (%) | OR | 95% CI | p-value |
Blood transfusion / Переливание крови | |||||
Yes / Да | 51 | 25 (12.5) | 1.397 | 0.758‒3.592 | 0.735 |
No / Нет | 149 | 79 (39.5) | |||
Malaria Parasite / Малярийный паразит | |||||
Yes / Да | 101 | 54 (27.0) | 1.396 | 0.748‒2.946 | 0.694 |
No / Нет | 99 | 50 (25.0) | |||
Typhoid / Брюшной тиф | |||||
Yes / Да | 73 | 32 (16.0) | 0.765 | 0.286‒2.834 | 0.086 |
No / Нет | 127 | 72 (36.0) | |||
Residential area / Местность проживания | |||||
Rural / Село | 44 | 28 (14.0) | 2.503 | 0.964‒4.692 | 0.085 |
Urban / Город | 156 | 76 (38.0) | |||
Nearness to bush / Близость к зарослям кустарника | |||||
Yes / Да | 82 | 43 (21.5) | 0.981 | 0.465‒1.694 | 0.964 |
No / Нет | 118 | 61 (30.5) | |||
Closeness to stagnant water or uncovered gutter / Близость к стоячей воде или открытому водостоку. | |||||
Not close / Далеко | 147 | 79 (39.5) | 1.645 | 0.786‒2.846 | 0.648 |
Very close / Очень близко | 53 | 25 (12.5) | |||
Use of Mosquito repellent / Использование репеллента от комаров | |||||
Frequently / Часто | 84 | 40 (20.0) | 0.999 | 0.740‒1.545 | 0.356 |
Rarely / Редко | 87 | 48 (24.0) | |||
Never / Никогда | 29 | 16 (8.0) | |||
Use of Mosquito Net / Использование москитной сетки | |||||
Yes / Да | 77 | 70 (35) | 0.196 | 0.076‒0.236 | 0.142 |
No / Нет | 123 | 6 (3) | |||
Total / Всего | 200 | 104 (52) | |||
Note. *Statistically significant (p < 0.05); OR ‒ odds ratio; CI ‒ Confidence Interval.
Примечание. *Статистически значимо (p < 0,05); OR ‒ отношение шансов; CI ‒ доверительный интервал.
Table 3. Prevalence of anti-WNV IgM antibodies in relation to risk factors among febrile patients attending selected hospitals in the Ilorin metropolis
Таблица 3.Частота выявления антител класса IgM к вирусу Западного Нила в зависимости от факторов риска среди пациентов с лихорадкой в отдельных больницах мегаполиса Илорин
Risk Factors / Факторы риска | n | IgM +Ve (%) | OR | 95% CI | p-value |
Blood transfusion / Переливание крови | |||||
Yes / Да | 51 | 6 (3.0) | 1.941 | 0.364‒4.642 | 0.886 |
No / Нет | 149 | 6 (3.0) | |||
Malaria Parasite / Малярийный паразит | |||||
Yes / Да | 101 | 7 (3.5) | 1.86 | 0.570‒2.962 | 0.780 |
No / Нет | 99 | 5 (2.5) | |||
Typhoid / Брюшной тиф | |||||
Yes / Да | 73 | 3 (1.5) | 0.782 | 0.389‒2.780 | 0.074 |
No / Нет | 127 | 9 (4.5) | |||
Residential area / Местность проживания | |||||
Rural / Село | 44 | 1 (0.5) | 0.369 | 0.056‒2.856 | 0.220 |
Urban / Город | 156 | 11 (5.5) | |||
Nearness to bush / Близость к зарослям кустарника | |||||
Yes / Да | 82 | 5 (2.5) | 1.142 | 0.549‒3.758 | 0.861 |
No / Нет | 118 | 7 (3.5) | |||
Closeness to stagnant water or uncovered gutter / Близость к стоячей воде или открытому водостоку | |||||
Not close / Далеко | 147 | 10 (5.0) | 1.646 | 0.386‒8.468 | 0.64 |
Very close / Очень близко | 53 | 2 (1.0) | |||
Use of Mosquito repellent / Использование репеллента от комаров | |||||
Frequently / Часто | 84 | 7 (3.5) | 1.086 | 0.688‒2.684 | 0.301 |
Rarely / Редко | 87 | 2 (1.0) | |||
Never / Никогда | 29 | 3 (1.5) | |||
Use of Mosquito Net / Использование москитной сетки | |||||
Yes / Да | 77 | 6(3) | 1.580 | 0.480‒7.608 | 0.406 |
No Нет | 123 | 6(3) | |||
Total / Всего | 200 | 12(6) | |||
Note. *Statistically significant (p < 0.05); OR ‒ odds ratio; CI ‒ Confidence Interval.
Примечание. *Статистически значимо (p < 0,05); OR ‒ отношение шансов; CI ‒ доверительный интервал.
Figures 2 and 3 display the logistic regression coefficients for the IgG and IgM positivity respectively. Significant positive coefficients were noticed on the risk factors as a predictor of seropositivity for IgM and IgG antibodies. Blood transfusion, malaria parasites, typhoid, rural residential areas and nearness to the bush are among the predictors with significant coefficients as depicted by the magnitude of the line for IgG antibodies (Fig. 2). Blood transfusion, typhoid, rural residential, proximity to the bush and uncovered drainage channel, mosquito repellent frequency and use of mosquito net were significant correlations to IgM positivity (Fig. 3). A negative correlation was noticed between the presence of malaria for IgG positivity and the use of mosquito nets for IgM positivity respectively.
Fig. 2. Logistic Regression Coefficients for Anti-WNV IgG Antibody Positivity.
Рис. 2. Коэффициенты логистической регрессии для положительного результата выявления анти-ВЗН класса IgG.
Fig. 3. Logistic Regression Coefficients for Anti-WNV IgM Antibody Positivity.
Рис. 3. Коэффициенты логистической регрессии для положительного результата выявления анти-ВЗН класса IgM.
Discussion
The study revealed the presence of WNV amongst the respondents as depicted by the IgM antibody prevalence of 6% (12/200). This prevalence is higher than the 2.9% reported in semi-arid zones in Nigeria [16], and 0.0% that was reported in Osun state Nigeria [17], but lower than 10.3% in Zambia [18] and 8.2% reported in North Dakota USA [19]. The difference in the IgM antibody prevalence could be attributed to the seasonality of sample collection where the cold period was posited to have a higher prevalence. However, a prevalence of 53% was reported in the southwestern city of Ibadan [20] in 1990 using complement-fixing antibodies. IgM antibody prevalence of 8.2% was reported in Ilorin while a slightly lower prevalence of 5.2% was noticed in the northern part of Nigeria [21]. This shows the silent transmission of WNV among the populace across different locations [16]. This study also reported a higher anti-WNV IgG antibody prevalence of 52% (104/200), this prevalence is higher than the 37.0% in Sudan, and 6.6% reported in Ilorin [21]. The differences may be due to the sample size, environmental conditions or geographical location.
In this study, the prevalence of West Nile Virus IgG antibodies varies across age groups, with the highest prevalence observed in the age group < 10 years (16.5%), while the age group 51‒60 years had the least prevalence (0%). A statistically significant association between age and West Nile Virus seropositivity rate was recorded. However, it’s important to note that the very low counts in some age groups may impact the validity. This study conforms with the research that delved into age-related patterns of West Nile Virus seroprevalence [22]. It was posited that age could be a significant factor, especially in areas with a substantial proportion of elderly individuals. Age-related differences in immunity and exposure could contribute to varying West Nile Virus seroprevalence rates. Contrary to this study, other studies [20, 23] reported a higher prevalence in the older age group. This study could not establish any statistically significant association between other variables including; gender, occupation, use of mosquito nets, formal education, use of mosquito nets, blood transfusion, co-infection of the malaria parasite, co-infection of typhoid fever, residential area, nearness to a bush, closeness to stagnant water and use of mosquito repellent and the occurrence of anti-WNV IgM and IgG antibodies signifying little or no contribution of these factors to the spread of the infection in this location. This could be related to the seasonality of this research or the significant level of IgG antibodies which depicts past infection amongst the participants. A statistically significant association between religion and anti-WNV IgM and IgG antibodies was noticed where the participants of the Islamic faith had a higher prevalence than the Christian faith. Religion, as a cultural and social determinant, can influence behaviours, practices, and exposure risks relevant in the context of infectious diseases. There are infrastructural differences/physical barriers in protection against the vector of the infectious agent. This correlates with a report [18] investigating the association between religion and West Nile Virus seroprevalence in a similar context. The importance of considering multiple factors, including environmental conditions and individual behaviors, to understand the dynamics of West Nile Virus seroprevalence was highlighted.
The blood transfusion history of respondents revealed an odd of 1.397 and 1.941 in participants with a history of blood transfusion than those without signifying increased risk among participants with blood transfusion history. This correlates with the report of blood as a possible transmission route of the virus [8]. Likewise, the odds of WNV IgG and IgM positivity amongst participants with the malaria parasite are 1.396 and 1.860 times higher than those without. This can be attributed to the increased risk associated with the shared vector of malaria and West Nile virus (i.e. mosquito) and the risk of false positivity by cross-reactivity. This is further supported by the magnitude of the coefficients in Figure 2 for IgG positivity.
A similar trend was noticed among participants in rural areas which are 2.503 times higher than those in urban areas. Participants in proximity to stagnant water or uncovered gutter are 1.645 and 1.646 times at risk of anti-WNV IgG and IgM positivity while nearness to bush had no significance for anti-WNV IgG positivity. Analysis of the odd for nearness to bush in relation to anti-WNV IgM antibodies showed 1.142 times higher risk compared to those that are not. The significant statistical expression based on odds ratio and CI correlate to the known factors that could improve or encourage the breeding sites of mosquitoes which can transmit the virus. Thus, public awareness of the route of transmission of the virus is encouraged alongside the advocation for the screening of blood samples for viral agents like West Nile virus to reduce localized transmission.
Conclusion
The findings of this study show the circulation of WNV in the study area. There is an urgent need for clinicians/physicians to include screening for the West Nile virus in cases of febrile patients before the commencement of treatment.
Funding. This study was not supported by any external sources of funding.
Acknowledgement. We acknowledge the Kwara State Ministry of Health for approving the research and Sobi specialist Hospital, Childrens’ specialist hospital, Civil Service Specialist Hospital, Cottage Hospital, Gaa Akanbi Area Healthcare, Hawau Ronke Memorial Medical Pathology Laboratory, Medicare Hospital, Olarenwaju Hospital, Omolola Hospital, Temitope Hospital and Yusjib Hospital for allowing access to their facilities and patients.
Conflict of interest. The authors declare no apparent or potential conflicts of interest related to the publication of this article.
Ethics approval. The study was conducted with the informed consent of the patients. The research protocol was approved by the Ethics and Health Research Board of the Ministry of Health, Ilorin, Kwara State, Nigeria (ERC/MOH/2023/02/091 dated 13th March, 2023).
Об авторах
Mutiat Busayo Odebisi-Omokanye
Университет Илорина
Автор, ответственный за переписку.
Email: odebisi.mb@unilorin.edu.ng
ORCID iD: 0000-0001-9825-3193
PhD (медицинская микробиология), старший преподаватель кафедры микробиологии
Нигерия, PMB 1515, ИлоринMuhammed Mustapha Suleiman
Саммитский университет Оффа
Email: Suleiman.muhammed@summituniversity.edu.ng
ORCID iD: 0000-0003-3275-1709
PhD (медицинская микробиология), преподаватель кафедры микробиологии
Нигерия, P.M.B. 4412, ОффаMariam Kehinde Sulaiman
Университет Илорина
Email: Sulaiman.km@unilorin.edu.ng
ORCID iD: 0000-0002-8295-7855
PhD (микробиология и клеточная биология), старший преподаватель кафедры медицинской микробиологии и паразитологии, Старый колледж медицинских наук
Нигерия, PMB 1515, ИлоринSidiq Abubakar Atolagbe
Университет Илорина
Email: ayinlatorla@gmail.com
ORCID iD: 0009-0007-2330-0232
аспирант кафедры микробиологии
Нигерия, PMB 1515, ИлоринСписок литературы
- Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013; 310(3): 308–15. https://doi.org/10.1001/jama.2013.8042
- Huhtamo E., Cook S., Moureau G., Uzcátegui N.Y., Sironen T., Kuivanen S., et al. Novel flaviviruses from mosquitoes: mosquito-specific evolutionary lineages within the phylogenetic group of mosquito-borne flaviviruses. Virology. 2014; 464-465: 320–9. https://doi.org/10.1016/j.virol.2014.07.015
- Chancey C., Grinev A., Volkova E., Rios M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015; 2015: 376230. https://doi.org/10.1155/2015/376230
- Hayes E.B., Komar N., Nasci R.S., Montgomery S.P., O’Leary D.R., Campbell G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 2005; 11(8): 1167–73. https://doi.org/10.3201/eid1108.050289a
- Calistri P., Giovannini A., Hubalek Z., Ionescu A., Monaco F., Savini G., et al. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol. J. 2010; 4: 29–37. https://doi.org/10.2174/1874357901004020029
- Mencattelli G., Ndione M.H.D., Rosà R., Marini G., Diagne C.T., Diagne M.M., et al. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl. Trop. Dis. 2022; 16(1): e0010075. https://doi.org/10.1371/journal.pntd.0010075
- Sampathkumar P. West Nile virus: epidemiology, clinical presentation, diagnosis, and prevention. Mayo. Clin. Proc. 2003; 78(9): 1137–43; quiz 1144. https://doi.org/10.4065/78.9.1137
- CDC. Blood Transfusion and Organ Donation (West Nile Virus); 2024. Available at: https://www.cdc.gov/west-nile-virus/causes/blood-transfusions.html#:~:text=Can%20I%20donate%20blood%20if,should%20tell%20your%20blood%20center
- Powell J.R. Modifying mosquitoes to suppress disease transmission: Is the long wait over? Genetics. 2022; 221(3): iyac072. https://doi.org/10.1093/genetics/iyac072
- Dahmana H., Mediannikov O. Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. Pathogens. 2020; 9(4): 310. https://doi.org/10.3390/pathogens9040310
- Pealer L.N., Marfin A.A., Petersen L.R., Lanciotti R.S., Page P.L., Stramer S.L., et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003; 349(13): 1236–45. https://doi.org/10.1056/NEJMoa030969
- Santini M., Haberle S., Židovec-Lepej S., Savić V., Kusulja M., Papić N., et al. Severe West Nile virus neuroinvasive disease: clinical characteristics, short- and long-term outcomes. Pathogens. 2022; 11(1): 52. https://doi.org/10.3390/pathogens11010052
- Hernandez Acosta R.A., Esquer Garrigos Z., Marcelin J.R., Vijayvargiya P. COVID-19 pathogenesis and clinical manifestations. Infect. Dis. Clin. North Am. 2022; 36(2): 231–49. https://doi.org/10.1016/j.idc.2022.01.003
- Monath T.P., Arroyo J., Miller C., Guirakhoo F. West Nile virus vaccine. Curr. Drug Targets Infect. Disord. 2001; 1(1): 37–50. https://doi.org/10.2174/1568005013343254
- CDC. West Nile virus. Clinical evaluation and disease; 2024. Available at: https://www.cdc.gov/west-nile-virus/hcp/clinical-signs/?CDC_AAref_Val=https://www.cdc.gov/westnile/healthcareproviders/healthCareProviders-ClinLabEval.html
- Ma’aji J.A., Olonitola O.S., Ella E.E. Seroprevalence of West Nile virus (WNV) infection among febrile patients attending selected hospitals in Kaduna state, Nigeria. Sci. Afr. 2020; 10: e00588. https://doi.org/10.1016/j.sciaf.2020.e00588
- Abdullahi I.N., Emeribe A.U., Ghamba P.E., Omosigho P.O., Bello Z.M., Oderinde B.S., et al. Distribution pattern and prevalence of West Nile virus infection in Nigeria from 1950 to 2020: a systematic review. Epidemiol. Health. 2020; 42: e2020071. https://doi.org/10.4178/epih.e2020071
- Abdullahi I.N., Emeribe A.U., Ghamba P.E., Omosigho P.O., Bello Z.M., Oderinde B.S., et al. Distribution pattern and prevalence of West Nile virus infection in Nigeria from 1950 to 2020: a systematic review. Epidemiol. Health. 2020; 42: e2020071. https://doi.org/10.4178/epih.e2020071
- Carson P.J., Borchardt S.M., Custer B., Prince H.E., Dunn-Williams J., Winkelman V., et al. Neuroinvasive disease and West Nile virus infection, North Dakota, USA, 1999–2008. Emerg. Infect. Dis. 2012; 18(4): 684–6. https://doi.org/10.3201/eid1804.111313
- Omilabu S.A., Olaleye O.D., Aina Y., Fagbami A.H. West Nile complement fixing antibodies in Nigerian domestic animals and humans. J. Hyg. Epidemiol. Microbiol. Immunol. 1990; 34(4): 357–63.
- Udeze A.O., Shittu H.K., Ashaka O.S., Jakkari A., Oyefolu A.O. Anti-West Nile virus immunoglobulin G and M profiles of patients with Pyrexia of unknown origin In Ilorin, Nigeria. Biokemistri. 2022; 34(3): 34432–40.
- Murgue B., Murri S., Triki H., Deubel V., Zeller H.G. West Nile in the Mediterranean basin: 1950–2000. Ann. N.Y. Acad. Sci. 2001; 951: 117–26. https://doi.org/10.1111/j.1749-6632.2001.tb02690.x
- Mease L.E., Coldren R.L., Musila L.A., Prosser T., Ogolla F., Ofula V.O., et al. Seroprevalence and distribution of arboviral infections among rural Kenyan adults: a cross-sectional study. Virol. J. 2011; 8: 371. https://doi.org/10.1186/1743-422x-8-371
Дополнительные файлы